Adobe Acrobat Sign CFR Part 11 Compliance
Coming Soon
You must submit a request to Office of Research IT (coming soon!) for all 21 CFR Part 11 needs. Note: This process may take up to 2 weeks to complete the setup and review pending no IRB modifications are needed. Please plan accordingly.
 Adobe Acrobat Sign is a digital signing software used to send documents online and collect electronic signatures remotely.
Adobe Acrobat Sign is a digital signing software used to send documents online and collect electronic signatures remotely.
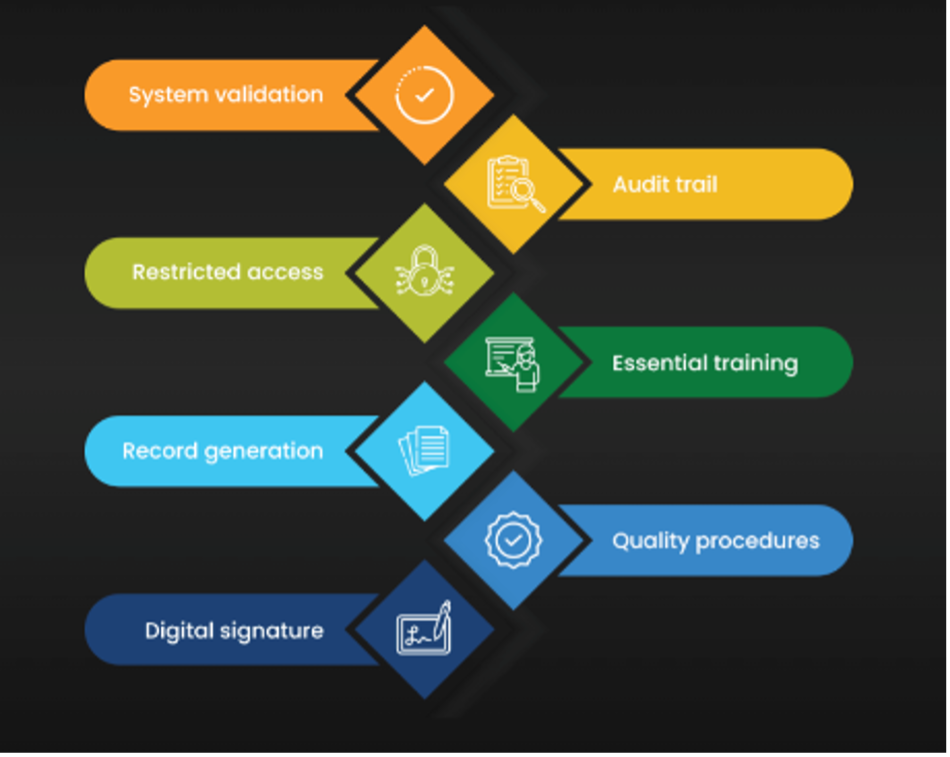
Adobe Acrobat Sign is the only University of Rochester approved 21 CFR Part 11 and HIPAA compliant option and is available for clinical research teams at the University of Rochester. Senders can upload documents to an electronic agreement, add fields for signature and date, and send the agreement to a specified recipient via email.
You must submit a request to Office of Research IT for 21 CFR Part 11 compliant set-up
A full-service review and set-up process will be required to use this service due to FDA audit requirements.
A few reminders:
- A mandatory 10-minute training and attestation will be required for all CFR Part 11 requestors before accounts will be granted.
- There is a $250 set-up fee for each unique CFR Part 11 request. eConsents may require a proposal depending on the requirements and complexity with associated fees.*
- There is a separate Adobe Acrobat Sign Part 11 account for sending agreements that require 21 CFR Part 11 compliance. No purchase of separate envelopes will be needed with Adobe Acrobat Sign.
- Acrobat Pro is not required to use Adobe Sign. You must upload a PDF or Word doc to use once setup and user access is granted.
- Adobe Acrobat Sign is not intended to be used as a document management system.
- New eSignature guidance will be coming soon.
*$250 setup fee does not include a license for Adobe Creative Cloud. Please see https://tech.rochester.edu/software/adobe/ for more information.
Non-CFR Part 11 signing tools
eConsent/CFR Part 11 signing tool
Requests for eConsent or CFR Part 11:
Questions for eConsent or CFR Part 11: