UR Medicine / Neurology / FSHD Research Center / Information for Patients and Families
Information for Patients and Families
The FSHD Research Center seeks to provide individuals with FSHD and their families with useful information about FSHD (FSH Dystrophy). Outlined below is a series of questions that clinicians are often asked regarding FSHD. We will strive to update this information as new data becomes available. Your feedback regarding the content of this page and suggestions about important topics you would like to see addressed, are welcome. Contact the FSHD Research Center at: FSHD-ResearchCenter@urmc.rochester.edu.
FSHD and Pregnancy
General Information about FSHD
What is FSHD?
Facioscapulohumeral Muscular Dystrophy, abbreviated either as FSH or FSHD, is an inherited disorder of muscle that causes progressive deterioration of muscle fibers resulting in muscle atrophy and weakness. It is one of many different forms of muscular dystrophy, each with a different genetic cause as well as different clinical symptoms, severity, and rate of progression. FSHD is the third most common form of muscular dystrophy after Duchenne muscular dystrophy and myotonic dystrophy. FSHD affects approximately 1 in 20,000 individuals.
How is FSHD Inherited?
FSHD type 1 (FSHD1) is dominantly inherited. This means that inheriting one defective copy of a segment of DNA from a parent with FSHD1 is sufficient to cause disease. The genetic defect in FSHD1 occurs on one end of chromosome number 4. All individuals carry two copies of their DNA in each cell but pass on a single copy to their offspring. A parent with FSHD1 will carry the FSHD1 genetic defect on one of two copies of chromosome 4 as illustrated in red in the diagram below. Therefore an affected parent has a 50:50 chance of passing on the defective copy to their child as illustrated in the diagram below.
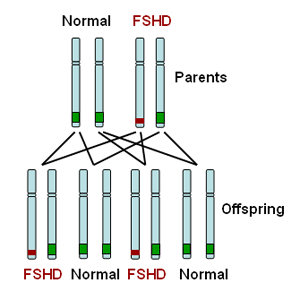
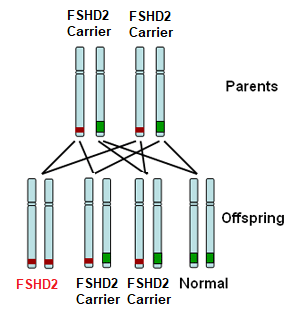
FSHD type 2 (FSHD2) is recessively inherited. This means that you must inherit one defective copy of a segment of DNA from each parent in order to have the disease. Each parent may not be affected with FSHD as they may just be a carrier for FSHD2. For those with FSHD2, there is a 1:4 (25%) chance of their child inheriting FSHD2.
There are exceptions, however, to this pattern of inheritance. In up to 30% of cases of individuals with FSHD, no signs of FSHD are detected in the parents and genetic testing shows no defect on chromosome 4. Such a situation, known as sporadic FSHD, means that the FSHD genetic defect occurred spontaneously in that individual. Such individuals can, in turn, pass this defect to their offspring.
What are the Symptoms of FSHD?
The name Facio (face) – Scapulo (shoulder blades) – Humeral (upper arm) indicates the particular distribution of the affected muscles which is characteristic early in FSHD. With time and depending on how severely an individual is affected, the leg muscles may become affected as well. This descending pattern of progression from face and shoulders to the legs is typical for most individuals.
The majority of individuals with FSHD typically start noticing symptoms in their teens but the range of the age at which symptoms start varies widely from early infancy, also called infantile FSHD, to late adulthood. In fact a fraction of individuals who have very mild weakness for which they have compensated may never notice any symptoms.
The typical initial complaint relates to weakness of the shoulders with winging of the shoulder blades and difficulty lifting arms overhead. At times, the initial symptom is tripping or slapping of the foot due to foot drop.
Does FSHD Affect Other Parts of the Body?
In the vast majority of individuals with FSHD, the symptoms are restricted to weakness of limb and trunk muscles. However, other parts of the body can be involved. This involvement is usually mild but in rare cases can cause severe disability:
- Heart: Individuals with FSHD have a higher incidence of cardiac arrhythmias due problems with the atria (upper chamber of the heart). This can result in episodes of rapid heart beat or palpitations. These types of arrhythmias are not life threatening and require treatment in less than 5% of individuals with FSHD.
- Difficulty Breathing: The breathing muscles are not involved in most patients with FSHD but it can be a problem in severely affected individuals, especially those who are confined to a wheelchair. The symptoms of breathing difficulty can be subtle and are most evident initially when a person is lying flat. The need to sleep on more than a couple of pillows, non-restful sleep, and chronic morning headaches may be indications of reduced lung capacity. A baseline measurement of lung capacity is important to obtain in individuals with severe disease and it should be repeated yearly. Lung capacity measurement should be obtained before surgery in all FSHD patients to alert the physicians about the possible need for post-operative respiratory support and the need to avoid sedatives that will depress breathing. In all, about 1% of patients with FSHD will have breathing problems that will require respiratory assistance.
- Retina: When careful studies of the retinal blood vessels are done in individuals with FSHD, about 60% will show minor abnormalities of the blood vessels. These minor abnormalities will not result in any problems in the vast majority of patients with FSHD. However, rarely, these retinal vessels become leaky and can cause bleeding and retinal detachment, a condition known as Coat’s disease. If there are significant abnormalities in the blood vessels, treatment with laser therapy will prevent the development of Coat’s disease and its associated loss of vision. It is now clear that this severe complication happens only in infantile onset FSHD or in patients with very large deletions (see below under section 7). Based on this information, patients with infantile onset FSHD or patients with large deletions should get a thorough dilated eye examination by an ophthalmologist. If abnormalities of the blood vessels are noted, they need to be followed closely.
- Hearing loss: High frequency hearing loss can be seen in FSHD. Like other non-muscular complications in FSHD, this is usually mild and requires no intervention except in a few patients where it is severe enough to necessitate the use of a hearing aid. Recognizing hearing loss is crucial in infantile onset FSHD as untreated hearing loss in a child can lead to delay in speech and language development. As with the retinal problems, patients with infantile-onset FSHD and those with large deletions are more susceptible to getting hearing loss that is severe enough to require a hearing aid.
Is Infantile FSHD Different From Other Forms of FSHD?
Infantile or childhood onset FSHD refers to occurrence of symptoms of patients at a very early. Infantile or childhood FSHD has the same genetic defect as all other forms of FSHD except that they tend to have a larger piece of missing DNA on chromosome 4 and typically have the most severe form of FSHD. In addition to having more severe muscle weakness, infantile FSHD patients also tend to have more severe, non-muscular complications that need to be addressed. Hearing loss needs to be recognized early and treated. The combination of unrecognized hearing loss and an unexpressive face due to severe facial muscle weakness can lead to the mistaken conclusion that the child is cognitively delayed. Speech therapy is important as severe weakness of the muscles of the mouth can result in speech problems and in problems eating. Infantile FSHD patients should also be carefully screened for retinal problems and breathing problems and treated accordingly. Some patients with infantile onset FSHD will also have cognitive problems.
What Can Someone With FSHD Expect As They Age?
In some forms of muscular dystrophy the course of the disease over time is uniform for everyone with that particular disease. The same cannot be said for FSHD. The course is extremely variable from person to person even within the same family. In general, over a lifetime, it is expected that about 20% of individuals with FSHD above the age of 50 will require the use of a wheelchair. The remaining 80% will have variable levels of disability ranging from very mild weakness to severe weakness.
It is difficult to project the course of the disease for any one person because of the unpredictable nature of the condition. In general, however, the earlier in life symptoms start, the more severe the disease is likely to be. Consequently, patients with FSHD1 who have very large deletions are predicted to have a more severe course. One other factor that affects disease severity is gender. Women with FSHD, as a group, tend to be more mildly affected and they are typically older when they start having symptoms of FSHD.
How is FSHD Diagnosed?
The diagnosis of FSHD is made by a Neurologist based on the pattern of muscle weakness that is seen on bedside examination. An EMG (electromyography) may be helpful in certain cases but it only confirms the presence of a muscular dystrophy and cannot differentiate between the different muscular dystrophies. The diagnosis can then be confirmed on blood DNA test, a test which has an accuracy of about 95 %. Genetic testing for FSHD using a blood sample is commercially available.
The genetic test consists of measuring the size of the DNA segment containing multiple copies of identical DNA sequences called repeats (Blue triangles in figure below). In the genetic testing process, molecular scissors (restriction enzymes) are used to cut the DNA on either side of the repeats. In healthy individuals both copies of chromosome 4 will have more than 10 copies of this repetitive sequence of DNA whereas in individuals with FSHD, one chromosome 4 will have only 1 and 9 copies. The readout from the genetic test gives two numbers, one from each copy of chromosome 4 (seen in red below) and corresponds to the size of the DNA segment, containing the repetitive sequences. An allele size of greater than 50 Kb represents a normal allele whereas an allele from someone with FSHD will be between 10 and 38 Kb.
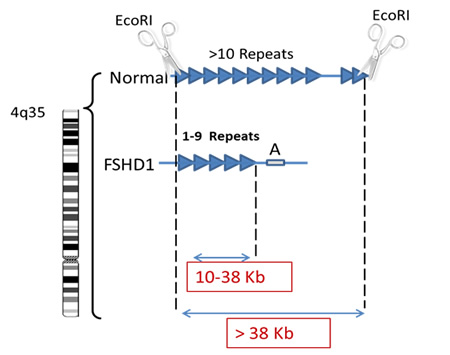
As discussed previously patients with very large deletions, resulting in a small residual piece of DNA tend to have more severe disease. So typically, patients whose contracted segment of the repeats measures between 10-17Kb will predictably have more severe muscle weakness and are more likely to have hearing and retinal vascular complications. On the other hand, having a contracted repeat segment between 20-38 Kb does not tell you much about prognosis since such patient vary from very mild to severe regardless of the size of the segment within that range.
If the DNA is negative for the FSHD defect, there could be one of two possibilities. The first is that the person may look like they have FSHD but, in fact, have another muscular dystrophy. It is not an uncommon for one form of dystrophy to look like another in its clinical presentation. In such a case, further blood testing in addition to a muscle biopsy may be needed to arrive at the proper diagnosis.
The second possibility is that the person has FSHD type 2 (FSHD2). This represents about 5% of individuals with FSHD. FSHD2 is inherited in a recessive manner, meaning you must inherit a DNA change from both your mother and your father. In FSHD2, there is no shortening of the chromosome 4 fragment below 38 kb, but there is “unwinding” of the DNA in the region on chromosome 4. This unwinding of the DNA is caused by a mutation on another gene. There are several genes identified on other chromosomes that can cause this, but SMCHD1 is the most common gene. Genetic testing for FSHD2 has become more widely available at testing laboratories to confirm this diagnosis.
What Causes FSHD?
Research published in 2010 identified a unifying cause of FSHD. The bottom line is that there is a gene contained within the repeats on chromosome 4, called DUX4, that is usually turned off and therefore not functional in mature cells. However, in patients with FSHD this gene is turned on in the muscle cells and the protein produced by this gene is toxic to muscle cells. The turning on of this normally dormant gene is the cause for FSHD1 and FSHD2 even though the genetic defect that leads to this different for each:
- FSHD1: The tip of chromosome 4 has a sequence of repetitive identical pieces of DNA, also called repeats. Typically individuals without FSHD have between 10-100 repeats on each copy of their chromosome 4. Each repeat contains the same sequence for the DUX4 gene. When the number of repeats is 10 or greater, the DNA containing this gene is tightly bound and the DUX4 gene is silenced. However, when you lose repeats and are down to 1-9 repeats, the DNA structure is loosened (Figure: unwound green line of the 1-9 repeats) and the DUX4 gene is turned on. This will only occur, however, when there is a particular DNA sequence called the A sequence, after the last repeat which stabilizes the RNA produced from the DUX4 DNA. The RNA is a copy of the DNA message that is then used to make the corresponding DUX4 protein.
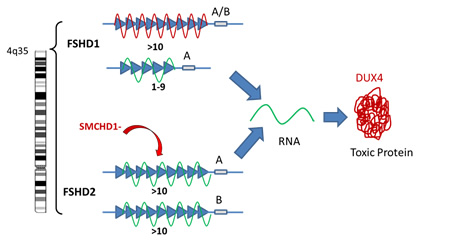
- FSHD2: In patients with FSHD2, the number of repeats on chromosome 4 is normal and yet the structure of the DNA is loosened by the reduction in the number of chemical bonds (called methylation) between the strands of the DNA. In about 80% of patients with FSHD2, we now know that this reduction in methylation bonds that loosens the DNA structure, is caused by a mutation in a gene on chromosome 18 called SMCHD1 (Figure: indicated as SMCHD- above). The protein produced by this gene is responsible for regulating how tightly bound DNA is on chromosome 4. Like FSHD1, if this loosening happens in the setting of the A distal sequence, once again, the DUX4 gene is turned on producing its toxic protein.
In summary in both FSHD1 and 2 the normally dormant DUX4 gene is turned on and the resulting protein is toxic to muscle fibers. In FSHD1 this happens because you lose a large number of repeats which loosens the DNA structure whereas in FSHD2 the DNA is loosened because another gene that produces a protein responsible for keeping the DNA tightly bound is mutated and the resulting protein is dysfunctional.
Are There Any Treatments for FSHD
There are no known treatments that will reverse the muscle weakness and wasting in FSHD. However, development of a targeted treatment that reverses the specific cause of muscle weakness and wasting is now possible as the underlying cause for FSHD has been identified. Research is now focused on findings ways to practically and safely target the DUX4 protein production or block its effects on muscle cells. Such an intervention may take several years to develop and test before clinical trials are started. In addition, there are a number of pharmaceutical companies working on treatments that can boost muscle regenerative capacity. Such an intervention may help restore muscle strength but it does not deal with the underlying cause of the muscle degeneration.
Although there are no treatments as yet that reverse muscle weakness in FSHD, a lot can be done to maximize function and minimize complications from the disease: Management of Limb weakness: Orthotic devices such as ankle- foot and knee-ankle-foot orthoses (braces) are helpful in preventing falls and improving the gait of individuals with foot drop and weakness of knee extension. Bracing to help shoulder mobility is impractical and ineffective but surgical scapular fixation, in selected individuals, will improve arm range of motion.
- Role of exercise: Unlike some forms of muscular dystrophy, there is no evidence that exercise is detrimental in FSHD. Exercise can strengthen muscles that are not severely affected and will improve the overall aerobic capacity of an individual. However, there are some important rules of thumb to keep in mind:
- Safety: the type of exercise must be tailored to an individual disability to minimize the risk of injury.
- Overuse: avoid vigorous exercise of joints surrounded by weak muscles because they are more susceptible to overuse injury.
- Less weight/resistance, more repetition: exercise with heavy weights and high resistance are more likely to injure muscle fibers.
- Pain: if it hurts, then it is too much.
- Treatment of pain: Many patients with FSHD develop chronic pain related to overuse of joints that are made lax by weak surrounding muscles. Common areas of pain include the shoulders and upper back, the lower back in association with the increased curvature of the spine (lordosis), and the knees. Management of pain should include a combination of physical therapy, stretching and the use of medications.
- Physical therapy: Physical therapy is critical in maintaining joint mobility, stretching and strengthening muscles and in preventing and improving chronic pain. A guide to physical therapy in FSHD written by our senior physical therapist, Shree Pandya, and others was published by The FSH Society.
- Treatment of breathing problems: As described in section 2, significant reduction in lung capacity in patients with FSHD is uncommon but more likely to be observe in those with more with more severe disease. Patient with symptoms of non-restful sleep, morning headaches, the need to prop oneself on two or more pillows when sleeping, and excessive short of breath with minimal exertion may be indications of reduced lung capacity. Such patients should undergo evaluation of their lung capacity (pulmonary function test). About 1% of individuals with FSHD develop significant symptoms related to a reduced lung capacity. The initial intervention in such patient is the use of a BiPAP machine typically at night. The BiPAP machine is a portable device that fits on a nightstand and generates intermittent pressure. The pressure, transmitted to the patient’s lungs through a face or nasal mask, helps them take a deeper breath. If the lung capacity is severely reduced, BiPAP may not adequately compensate for the patient’s breathing problems. In such cases, a ventilator that provides a fixed volume of air to the patient’s lungs, typically through a tracheotomy, may be required.
- Retinal and hearing problems: As described in sections 2 and 3, screening for retinal problems in infantile FSHD and those with large deletions in FSHD1 is important to try to prevent retinal hemorrhage that can result in blindness. Similarly screening for hearing loss in the same population may help prevent delayed or impaired language development in children.