Research News
Eric Small's Research Suggests a Cancer Protein Could Be at the Heart of Cardiac Scarring and Disease
The associate professor of Medicine and his colleagues found that the tumor suppressor protein p53 might play an important role in both. Supported in part by the CTSI, the research shows that too much p53 may speed progression of a heart rhythm disease, while too little p53 could lead to scarring after cardiac injury.

2025 Pilot Awardees Will Tackle Everything from Microplastics to Contraception
Thursday, May 22, 2025
The Department of Medicine funds promising research, quality, and educational initiatives through its Research and Education Pilot Awards program. Awardees receive $20,000-$40,000 for basic, clinical, or translational research or educational scholarship projects.
Congratulations to this year’s awardees, who will kick their projects off on July 1:
Optimizing cardiac imaging in women with early-stage breast cancer
 Approximately 20% of breast cancers overexpress human epidermal growth factor 2 (HER2). While HER2-targeted therapies improve survival, they also increase the risk of heart failure. Current guidelines recommend that all women receive cardiac imaging every three months during treatment, regardless of their risk of cardiotoxicity. Farhan Bajwa, MD, assistant professor of Cardiology, will study whether tailoring the frequency of cardiac imaging to each patient’s baseline risk of cardiotoxicity (prior to cancer treatment) reduces harm and waste.
Approximately 20% of breast cancers overexpress human epidermal growth factor 2 (HER2). While HER2-targeted therapies improve survival, they also increase the risk of heart failure. Current guidelines recommend that all women receive cardiac imaging every three months during treatment, regardless of their risk of cardiotoxicity. Farhan Bajwa, MD, assistant professor of Cardiology, will study whether tailoring the frequency of cardiac imaging to each patient’s baseline risk of cardiotoxicity (prior to cancer treatment) reduces harm and waste.
Evaluating curriculum for hospitalists and residents in caring for patients with an eating disorder
 Adults who receive adequate care can recover from eating disorders, yet eating disorders have the second-highest mortality rate of all mental health disorders due to a lack of access to care. Adam Bracken, MD, assistant professor of Hospital Medicine and Pediatrics, will develop and evaluate a curriculum to train Hospital Medicine faculty and APPs, and Internal Medicine residents to diagnose and manage patients with eating disorders. The curriculum for faculty and APPs would include three one-hour sessions with an opportunity for a simulated patient experience. The resident curriculum is a two-week elective with rotations on inpatient units. Both curricula will be assessed iteratively using the Context, Input, Process, and Product evaluation model.
Adults who receive adequate care can recover from eating disorders, yet eating disorders have the second-highest mortality rate of all mental health disorders due to a lack of access to care. Adam Bracken, MD, assistant professor of Hospital Medicine and Pediatrics, will develop and evaluate a curriculum to train Hospital Medicine faculty and APPs, and Internal Medicine residents to diagnose and manage patients with eating disorders. The curriculum for faculty and APPs would include three one-hour sessions with an opportunity for a simulated patient experience. The resident curriculum is a two-week elective with rotations on inpatient units. Both curricula will be assessed iteratively using the Context, Input, Process, and Product evaluation model.
Using AI to improve clinicians’ communication with older patients with advanced cancer
 Ehsan Hoque, MD, professor of Computer Science, will develop and test the AI platform, SOPHIE, to help clinicians improve communication skills for serious illness conversations with advanced cancer patients. SOPHIE allows clinicians to practice conversations with a virtual patient and receive real-time feedback to improve clarity, empathy, and patient empowerment. Hoque and Epstein will create scenarios reflecting diverse clinical situations to enhance communication, particularly with underserved and low-literacy populations.
Ehsan Hoque, MD, professor of Computer Science, will develop and test the AI platform, SOPHIE, to help clinicians improve communication skills for serious illness conversations with advanced cancer patients. SOPHIE allows clinicians to practice conversations with a virtual patient and receive real-time feedback to improve clarity, empathy, and patient empowerment. Hoque and Epstein will create scenarios reflecting diverse clinical situations to enhance communication, particularly with underserved and low-literacy populations.
Characterizing the infusion of microplastics to patients undergoing cardiopulmonary bypass
 Cardiopulmonary bypass pumps, which are used in over 90% of cardiac surgeries, have many flexible and compressible components made of the synthetic polymer, polyvinyl chloride (PVC).
Cardiopulmonary bypass pumps, which are used in over 90% of cardiac surgeries, have many flexible and compressible components made of the synthetic polymer, polyvinyl chloride (PVC).
Mark Marinescu, MD, assistant professor of Clinical Medicine, Cardiology, will investigate if PVC or other synthetic polymers leach into patient blood during bypass support in the form of microplastics. Preliminary findings indicate that contamination from the cardiopulmonary bypass circuit is introduced into the priming solution despite using a pre-bypass filter. How these microplastics impact health is still unknown.
Comparing six-minute walk test to cardiopulmonary exercise test
 Nearly half of patients hospitalized with acute pulmonary embolism report breathlessness and functional limitation three months later. These patients often undergo cardiopulmonary exercise testing (CPET) to evaluate physiologic abnormalities during exercise, but this test does not replicate patients’ normal weight-bearing activities and is too strenuous to administer within three months of an acute pulmonary embolism. Dominick Roto, DO, assistant professor of Pulmonary Diseases and Critical Care, will investigate whether a six-minute walk test with incorporated CPET measurements can produce similar testing results as the traditional maximal-effort CPET. The six-minute walk test does not require strenuous exercise or expensive equipment and better replicates patients’ normal activity, suggesting it may be a safe and cost-effective alternative for early functional assessment of post-pulmonary embolism impairment.
Nearly half of patients hospitalized with acute pulmonary embolism report breathlessness and functional limitation three months later. These patients often undergo cardiopulmonary exercise testing (CPET) to evaluate physiologic abnormalities during exercise, but this test does not replicate patients’ normal weight-bearing activities and is too strenuous to administer within three months of an acute pulmonary embolism. Dominick Roto, DO, assistant professor of Pulmonary Diseases and Critical Care, will investigate whether a six-minute walk test with incorporated CPET measurements can produce similar testing results as the traditional maximal-effort CPET. The six-minute walk test does not require strenuous exercise or expensive equipment and better replicates patients’ normal activity, suggesting it may be a safe and cost-effective alternative for early functional assessment of post-pulmonary embolism impairment.
Impact of weight gain perceptions on hormonal contraceptive selection
 Hormonal contraceptives are highly effective, but women with overweight or obesity may be more likely to discontinue them due to weight gain concerns. Adnin Zaman, MD, assistant professor of Endocrine/Metabolism, will develop and validate a novel, patient-centered survey to assess women’s experience and perceptions of weight-related side effects of hormonal contraceptives. In the long term, this survey could serve as a clinical tool to help providers tailor contraceptive counseling.
Hormonal contraceptives are highly effective, but women with overweight or obesity may be more likely to discontinue them due to weight gain concerns. Adnin Zaman, MD, assistant professor of Endocrine/Metabolism, will develop and validate a novel, patient-centered survey to assess women’s experience and perceptions of weight-related side effects of hormonal contraceptives. In the long term, this survey could serve as a clinical tool to help providers tailor contraceptive counseling.
Researchers Have Their Fingers on the Pulse of the Annual Heart Rhythm Society Meeting
Wednesday, May 14, 2025

Left to right: Valentina Kutyifa, MD, PhD, Ilan Goldenberg, MD, David Huang, MD,
Wojciech Zareba, MD, PhD, Claudio Schuger, MD, (of Wayne State University)
and Mehmet Aktas, MBA, MD
Cardiology researchers were among the more than 10,000 heart rhythm clinicians, allied professionals, scientists, and researchers who attended the Heart Rhythm Society’s annual meeting this year.
The meeting featured 200 educational sessions led by more than 2,000 experts, including:
Mehmet Aktas, MBA, MD, of Cardiology, presented a poster showing that the cancer drug, acalabrutinib, is associated with increased risk of atrial fibrillation in patients with lymphoproliferative disorders on behalf of lead author Saadia Sherazi, MBBS, MS, of Highland Hospital Medicine.
Ilan Goldenberg, MD, of Cardiology Heart Research, participated in a faculty debate arguing for the need to use risk stratification strategies prior to implanting a primary prevention implantable cardioverter device (ICD) in patients with ischemic or nonischemic heart failure.
Daniel Han, a student in the Medical Scientist Training Program, presented the poster “Non-Obese Patients with Metabolic Syndrome and Heart Failure Outcomes in ICD or Cardiac Resynchronization Therapy with Defibrillator (CRT-D) Patients in MEGA-MADIT,” a project that was mentored by Valentina Kutyifa, MD, PhD.
David Huang, MD, of Cardiology, presented the poster “Effects of Left Ventricular Assist Device (LVAD) Support on Recurrent Ventricular Arrythmias and ICD Therapies Among Advanced Heart Failure Patients: Comparing the PIVATAL and RAID Trials.”
 Valentina Kutyifa, MD, PhD, of Cardiology Heart Research, was part of a panel discussion on breaking down gender disparities in cardiovascular clinical trials leadership and hosted a research roundtable in the Fellows-in-Training Meet-the-Mentors session. She also had a late-breaking oral presentation, “Contemporary Outcomes of Non-Ischemic Cardiomyopathy Patients with Implanted Devices” related to BIO-LIBRA, a multicenter clinical study on non-ischemic cardiomyopathy, led by the University of Rochester in collaboration with Biotronik. Kutyifa also presented a poster “Risk of Heart Failure or Death in Men and Women with RBBB in MEGA-MADIT,” to provide information on outcomes of this cohort.
Valentina Kutyifa, MD, PhD, of Cardiology Heart Research, was part of a panel discussion on breaking down gender disparities in cardiovascular clinical trials leadership and hosted a research roundtable in the Fellows-in-Training Meet-the-Mentors session. She also had a late-breaking oral presentation, “Contemporary Outcomes of Non-Ischemic Cardiomyopathy Patients with Implanted Devices” related to BIO-LIBRA, a multicenter clinical study on non-ischemic cardiomyopathy, led by the University of Rochester in collaboration with Biotronik. Kutyifa also presented a poster “Risk of Heart Failure or Death in Men and Women with RBBB in MEGA-MADIT,” to provide information on outcomes of this cohort.
Alexei Nakonechnyi, PhD, and Dillon Dzikowicz, PhD, RN, PCCN, of Cardiology Heart Research, presented two posters, “Dynamic Risk Stratification for Progression to Persistent Atrial Fibrillation in Patients with Cardiac Implanted Electronic Devices,” and “Machine Learning for Predicting Progression to Persistent Atrial Fibrillation Using Point-in-Time Risk Stratification.”

Amole Ojo, MD, of Cardiology, presented the poster “Risk Factors and Outcomes Associated with Failed Implantable Cardioverter Defibrillator Shocks,” and hosted an oral abstract session exploring the role and outcomes of cardiac resynchronization therapy (CRT) and conduction system pacing among patients in common, specific scenarios. He also participated as a mentor in the Fellows-in-Training and the Medical Student, Intern, & Resident Meet-the-Mentors Roundtables. Ojo also co-hosted the HRS Board Review Course, a refresher on electrophysiology fundamentals for physicians and fellows preparing for certification or recertification.
Wojciech Zareba, MD, PhD, of Cardiology and Cardiology Heart Research, presented two posters, “Clinical Phenotype of Variants of Uncertain Significance in Long QT Syndrome Patients by Genotype,” and “Clinical, Electrocardiographic, and Cardiac Magnetic Resonance Imaging Risk Factors Associated with Ventricular Tachyarrhythmias in Nonischemic Cardiomyopathy (MARVEN Study).”
Strategic Plan: Expanding Robust Research to Achieve Breakthrough Discoveries
Wednesday, April 16, 2025
This is part of a series highlighting the Department of Medicine 2024-2028 Strategy and Action Plan, its five pillars for success, and progress toward meeting the goals.

Collaborative research that advances discovery is essential for success, and the DOM is committed to securing new funding to support further exploration.
To attain this goal, outlined in the Strategic Plan, the priorities are:
- Expand basic, translational, clinical, medical education, and DEI research through interdisciplinary teams across diverse disciplines;
- Increase accruals in clinical trials and expand clinical research across the lifespan of diverse populations; and,
- Enhance our national and international reputation via interdisciplinary research and collaboration.
 Laura Calvi, MDLaura Calvi, MD
Laura Calvi, MDLaura Calvi, MD Valentina Kutyifa, MD, PhDValentina Kutyifa, MD, PhD“We are focused on providing internal teams the tools and resources to prepare, submit and attain new grant awards,” said Valentina Kutyifa, MD, PhD, vice chair for Clinical Research. “New grants will lead to more groundbreaking scientific discoveries that improve patient care.”
Valentina Kutyifa, MD, PhDValentina Kutyifa, MD, PhD“We are focused on providing internal teams the tools and resources to prepare, submit and attain new grant awards,” said Valentina Kutyifa, MD, PhD, vice chair for Clinical Research. “New grants will lead to more groundbreaking scientific discoveries that improve patient care.”
Efforts to expand research through interdisciplinary teams are already yielding results:
- New grant awards: In fiscal year 2024, DOM was awarded $41.3 million in grants, with $7.6 million in new awards. Researchers from all divisions submitted 187 proposals and 67 projects were awarded. There are 488 active externally sponsored projects, 294 of which are clinical trials.
- Biostatistical Shared Resource: Launched in 2022, this program supports mentored faculty and trainee researchers who do not have funding to cover their activities. Areas of support include study preparation and pre-study planning, sample size, basic statistics, multivariate regression, non-parametric tests, correlations, basic survival analyses and database management. Laura M. Calvi, MD, vice chair for Basic and Translational Science, is spearheading the biostatistical shared resource program.
- Division faculty research delegates: Delegates representing each division’s basic and clinical research identify areas of improvement, and design and launch new initiatives to expand current research efforts.
- New research website: launched in 2024, provides a centralized platform for showcasing research, publications, collaborations, research teams, and resources. It is designed to be a resource for internal team members, external collaborators and the broader community. The research leadership team is supported by Stefanie Fingler, MBA, director of Research Operations, and Kari Steinmetz, assistant director of Clinical Research.
Activities also are underway to increase clinical trial accruals:
- Initial study feasibility process: In January, leaders unveiled a new standard operating procedure for feasibility of clinical trials as part of a new, comprehensive clinical trials platform. Its primary mission is to achieve translation through clinical trials, with the goal of creating a uniform framework to identify innovative studies that align with the missions of DOM’s various divisions.
- OnCore use policy: To harmonize OnCore use across the department, active studies must be entered into the clinical trial management system. This allows for accurate tracking of study status, subject recruitment, and patient demographics.
- Ongoing study feasibility process: A pilot initiative is in place to assess ongoing study feasibility. All DOM clinical research protocols will be reviewed semi-annually and annually to ensure adequate scientific progress.
Another priority is to expand collaborations and research abroad. New initiatives in this area include the Uganda Program, led by Paul R. Bohjanen, MD, PhD, chief of Infectious Diseases, with the aim to develop a Global Health Program focusing on infectious diseases research and treatment. And, a program aimed at initiating new scientific and educational programs with the National University of Sciences & Technology in Islamabad, Pakistan is being led by Bilal Ahmed, MD, FACP, FRCP, of Internal Medicine.
Research Leadership Team Update
Friday, February 14, 2025
There have been many recent federal policy changes that have left us all with questions and concerns. The Department of Medicine is committed to providing clear, coordinated, and consistent guidance on these changes, in alignment with the University of Rochester.
The University has launched a dedicated website to provide our community with updates and guidance on federal policy changes. The site includes:
- FAQs on research, immigration enforcement, and data privacy and legal protections
- The latest University messages on executive orders and announcements
- Guidance for researchers
- Web form for community members can submit questions.
- A new Policy Updates for Researchers web page
- Additional guidance on immigration enforcement and University protocols, including legal parameters, and rights and responsibilities for students, employees, and University officials when interacting with immigration officers.
You are encouraged to share these resources with colleagues who may receive questions or need this information in their roles.
We will continue to provide updates as we receive them. If you have any questions, concerns or ideas to share, related to recent changes and impacts on research specifically, please reach out to us: Valentina Kutyifa, MD, PhD, Laura Calvi, MD, or Stefanie Fingler (Stefanie_Fingler@URMC.Rochester.edu).
Three Faculty Earn Research, Teaching Awards
Wednesday, February 5, 2025

Brendan Guercia, MD

Erika Drury, MD

Rebecca Levy, MBBCh
Nephrology and Hematology/Oncology faculty were awarded research and teaching grants to advance care and education of the next generation of physicians.
Nephrology’s Rebecca Levy, MBBCh, received the 2025 UR CTSI K12 (formerly KL2) Scholar Award to examine outcomes for adolescents and young adults with chronic kidney disease and explore potential interventions to reduce unnecessary hospitalizations. The award provides 75 percent FTE support for two years.
Erika Drury, MD, of Nephrology, was selected as the 2025-2027 Ritchie Teaching Fellow. She will create a cardiovascular-kidney-metabolic health curriculum for the Nephrology Fellowship. The two-year award provides 10 percent FTE support.
Brendan Guercio, MD, of Hematology/Oncology, received a $250,000 Career Development Award from the Bladder Cancer Advocacy Network (BCAN.) He will investigate whether a healthy diet can help the immune system’s ability to fight advanced bladder cancer when coupled with the newest therapy, enfortumab, vendotin, and pembrolizumab (EVP). Guercio previously won a BCAN Young Investigator Award and has already shown that a nutritious diet enhancing the gut microbiome, along with immunotherapy, may lead to better bladder cancer control.
Olga Astapova Honored with Early Career Innovator Award
Thursday, January 30, 2025
 Olga Astapova, MD, PhD, assistant professor of Endocrinology, Diabetes & Metabolism, accepted the inaugural Molecular Human Reproduction (MHR) Early Career Innovator Award for “research that exemplifies the innovation, dedication, and rigor that propel the field of reproductive sciences forward.” The honor recognizes young researchers who publish original research articles as part of the Early Career Innovator Series.
Olga Astapova, MD, PhD, assistant professor of Endocrinology, Diabetes & Metabolism, accepted the inaugural Molecular Human Reproduction (MHR) Early Career Innovator Award for “research that exemplifies the innovation, dedication, and rigor that propel the field of reproductive sciences forward.” The honor recognizes young researchers who publish original research articles as part of the Early Career Innovator Series.
Kari Steinmetz Wins CTSI Shark Tank Funding Competition
Wednesday, January 22, 2025
 CTSI held its Translational Science Shark Tank event in October, with teams developing solutions to seemingly intractable barriers to recruitment in clinical research.
CTSI held its Translational Science Shark Tank event in October, with teams developing solutions to seemingly intractable barriers to recruitment in clinical research.
Kari M. Steinmetz, BA, COA, assistant director of Clinical Trials, and her team, presented creation of an Open Research Round Table Committee to enhance collaboration between research teams and participant recruitment groups. Following the competition, Steinmetz was asked to conceptualize her idea and secured CTSI funding to pursue it.
“With these resources, we aim to eliminate communication barriers that frequently impede clinical-research recruitment” Steinmetz said. “Through a standardized system, we hope to streamline collaboration among research-team members while enhancing participant engagement.”
Communication barriers between researchers and recruitment teams frequently slow the enrollment process in clinical trials, resulting in inefficient recruitment and missed enrollment targets. By identifying barriers to recruitment and discussing potential solutions, the round table committee will help meet research-enrollment targets and better engage research participants.
The committee will consist of coordinators, principal investigators, and study participants, and initially be piloted in Infectious Diseases before expansion across all divisions. The model could also be applied to community engagement, allowing potential participant recruitment groups to contribute ideas.
“My hope is that we can turn this pilot into a long-term project that expands from an internal process to a community effort,” Steinmetz said. “For example, in the community, we could discuss a barrier such as vaccine hesitancy. Researchers would have the opportunity to learn more about the reasons behind this hesitancy and to explain the safety of vaccines.”
New Procedures Launched to Bolster Clinical Trials
Wednesday, January 22, 2025
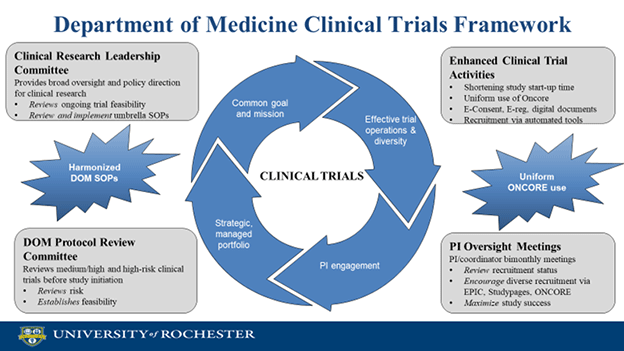
A new standard operating procedure (SOP) for Department of Medicine investigators was recently introduced to ensure feasibility of clinical trials as part of a new, comprehensive clinical trials platform. Its primary mission is to achieve translation through clinical trials, with the goal of creating a uniformed framework to identify innovative studies that align with the missions of DOM’s various divisions.
“We are launching a novel, innovative clinical trials framework to promote a strategic, managed clinical trials portfolio, effective trial operations and diversity, in alignment with the DOM Strategic Plan,” said Valentina Kutyifa, MD, PhD, vice chair for Clinical Research. “Most importantly, processes for start-up, feasibility, and conduct of clinical trials are being harmonized and modernized across our divisions to set up our investigators for success.”
With this streamlined process, new studies will be identified that are feasible and advance the institution’s strategic priorities. Last week, URMC CEO David Linehan, MD, cited his goal of building an “absolute powerhouse for clinical trials and clinical investigations.”
The DOM is at the forefront of this effort, with more than $247.7 million in research funding is the largest across SMD. This is the first of the DOM Clinical Trials Umbrella procedures aiming to achieve these goals.
The SOP’s scope covers clinical research protocols that prospectively enroll randomized and non-randomized human subjects, including sponsored and investigator-initiated research, clinical trials and FDA-regulated studies.
Key elements of the new DOM clinical trials framework include a Clinical Research Leadership Committee, harmonized organizational structure and mission statement, harmonized use of OnCore across divisions, a Protocol Review Committee and enhancing study start-up activities including IRB support. Other elements include advancing the use of e-consent and electronic study documentation, ongoing feasibility review, PI engagement, improved oversight and training, and competitive staff recruitment, training and retention.
The feasibility assessment of new clinical trials encompasses seven steps: sponsor inquiry, go/no go check, completion of a new, standardized feasibility worksheet, formal risk assessment through OCR, score calculation, protocol review by the DOM Protocol Review Committee and decision.
The next initiative is focusing on harmonizing the use of OnCore across all divisions. DOM hopes to soon implement its usage in all studies, currently at about 80 percent for DOM sponsored studies across all divisions.
“This SOP marks the beginning of a unified approach across all 15 divisions of medicine, providing a clear framework to align clinical trials with our strategic goals and ensuring swift and successful execution of studies that drive our mission forward,” said Stefanie Fingler, MBA, director of Research Operations.
Kari Steinmetz, MBA, assistant director of Clinical Trials, said, “With almost three decades of experience across various departments at the University of Rochester, I’ve seen firsthand how clear, consistent and well-communicated SOPs can serve as a vital tool in driving efficiency, consistency and quality across all levels of an organization. This SOP will help us reduce inefficiencies, foster communication and ultimately drive the success of our organization.”
The new SOP is a pilot that will be evaluated at the end of the year. For questions, contact the Research Leadership team.
URMC Department of Medicine Clinical Trials Framework
Department of Medicine Sponsors Research in DEI and Health Equity Symposium
Wednesday, January 8, 2025
On February 28, 2025, the Department of Medicine invites members of the University and surrounding communities to attend the 3rd annual Research in DEI and Health Equity Symposium. This year’s symposium places a special emphasis on research addressing community outreach and engagement, including but not limited to community-based participatory research, community health literacy, outreach efforts to Rochester community, LGBTQ+ care, engaging patient voice in clinical research and efforts to increase diversity in medical education. We are thrilled to welcome Dr. Wally Smith from Virginia Commonwealth University as our keynote speaker.
For questions about the registration site or process, please email: cmeregistration@urmc.rochester.edu
Jennifer Anolik Named Chief of Allergy, Immunology and Rheumatology Division
Monday, January 6, 2025
 Internationally renowned physician scientist Jennifer H. Anolik, MD, PhD, was named chief of the Allergy, Immunology and Rheumatology (AIR) Division in the Department of Medicine, after serving as interim chief since 2021.
Internationally renowned physician scientist Jennifer H. Anolik, MD, PhD, was named chief of the Allergy, Immunology and Rheumatology (AIR) Division in the Department of Medicine, after serving as interim chief since 2021.
“I am delighted that Jen has agreed to continue leading our AIR division as chief,” said Ruth O’Regan, MD, chair of Medicine. “The division is in great hands under her leadership and will continue to benefit from her experience and expertise as a clinician, educator and researcher. I am extremely grateful to her for serving as interim chair over the past few years.”
A faculty member for 22 years, Anolik also serves as associate chair for research in the Department of Medicine, professor of Medicine, Pathology, and Microbiology and Immunology, and director of the Internal Medicine Physician Scientist Training Program.
Anolik earned her MD and PhD at the University, completed her residency in Internal Medicine in 1999 and her fellowship in Rheumatology in 2002, after which she joined the faculty.