Projects
Participation of DC-STAMP in Inflammatory Arthritis
Bone remodeling is a dynamic and complex process finely orchestrated by cell interactions among osteoblast, T, B and natural killer cells. The first phase of bone remodeling requires activation of monocytes/macrophages by M-CSF and the receptor activator nuclear factor-kappa B ligand (RANKL) to initiate cell-cell contact and induce the formation of multinucleated osteoclasts (OCs).
DC-STAMP, a transmembrane protein, is essential at early stages of cell-cell fusion and OC differentiation. Although osteoclast precursors lacking DC-STAMP form mononuclear osteoclasts (mOCs) in vitro, they are still able to dissolve bone. Despite DC-STAMP being expressed in different cell types including dendritic cells, T cells, mononuclear and stromal cells, there are scarce studies showing its role in the immune response. Thus, the focus our laboratory is to:
- Elucidate the contribution of DC-STAMP in bone remodeling in TNF Tg and K/BxN mice, two inflammatory arthritis models
- Evaluate the therapeutic effect of neutralizing DC-STAMP antibodies in a humanized mouse model of rheumatoid arthritis and psoriatic arthritis
- Investigate whether DC-STAMP modulates tumor immunity using a mouse melanoma model.
Our main goal is to underline the cellular and molecular pathways that are affected by DC-STAMP expression and/or activation during the onset of RA in TNF Tg and NODxKRN mouse model. We recently showed that the absence of DC-STAMP in TNF Tg mouse model decreases bone resorption and migration of osteoclast precursors to inflamed synovium.
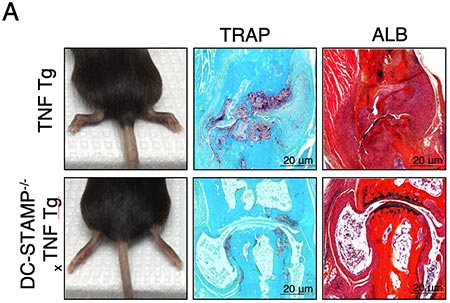
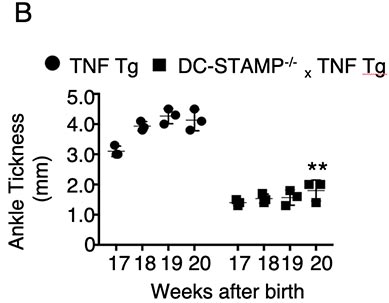
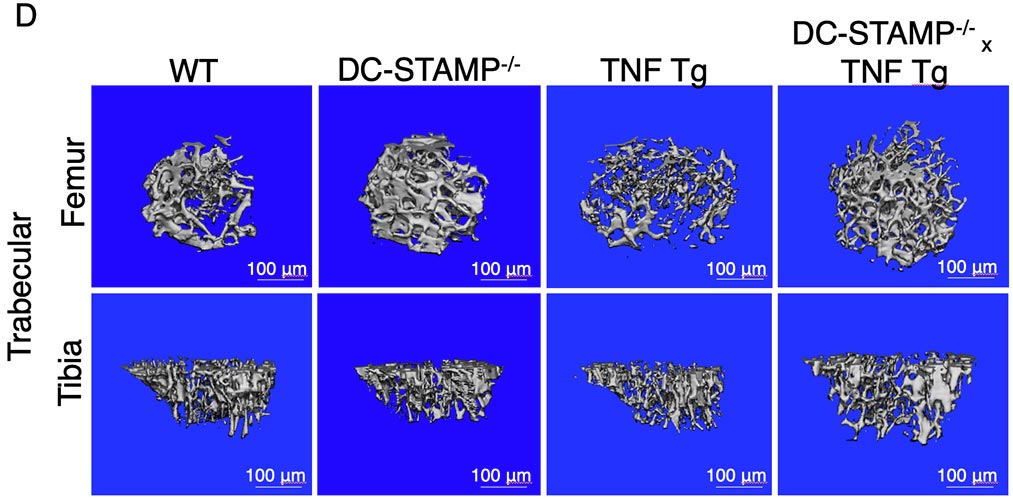
Figure Legend. The absence of DC-STAMP ameliorates RA in TNF Tg mice.
TNF Tg mice show visibly swollen joints (A-left panels), accumulation of TRAP+ osteoclasts, considerable inflammatory cell infiltration and cartilage damage (A-right panels) compared with DC-STAMP-/-x TNF Tg mice, which correlate with a significant increase of ankle thickness (B), higher percentage of TRAP+ area(C), and low bone density (D). Representative results of 5-month old TNF Tg and DC-STAMP-/-x TNF Tg mice are shown.