URMC / Labs / Fowell Lab / Projects / Execution of CD4 Effector Function
Execution of CD4 Effector Function
The molecular control of CD4 differentiation into functionally distinct effector lineages has been extensively studied but the fate of the effector T cells once they leave the lymph node is less well understood. We are interested in how the inflammatory milieu at sites of infection and autoimmune attack may modify the effector function of CD4+ T cells. Our main hypothesis is that while CD4 T cells may be programmed for function, the efficiency of executing that function will be controlled by the type and extent of inflammation at the pathogenic site. Function may be modified by the degree of recruitment or retention in the tissue, provision of the signals required to support high level cytokine production and/or reprogramming in light of the potential for functional plasticity.
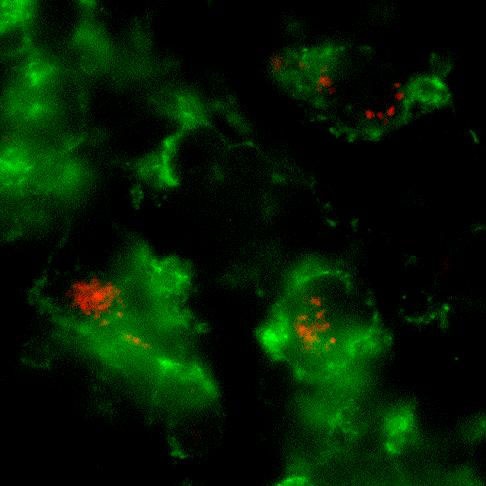
Whole mount microscopic image of PKH26-labeled L. major inside CD11b+ macrophages in the infected ear.
Our previous work with Leishmania major revealed the functional editing of the available cytokine repertoire at the infection site (Katzman et. al., 2008, JCI). Current studies use advanced imaging techniques to visualize defined effector populations within inflamed tissue and their interaction with antigen-bearing cells. The ear dermis provides a tractable site for intra-vital 2-photon microscopy and fluorescent tags on effector cells and Leishmania enable the analysis of cellular interactions. We are also developing new genetic tools to measure effector cell dwell time in inflamed tissue and to follow the functional fate of Th1 and Th2 cells in different inflammatory settings.