Clinical Studies
Title: A descriptive study of the pathogenesis of acute influenza in adults, children, and the elderly.
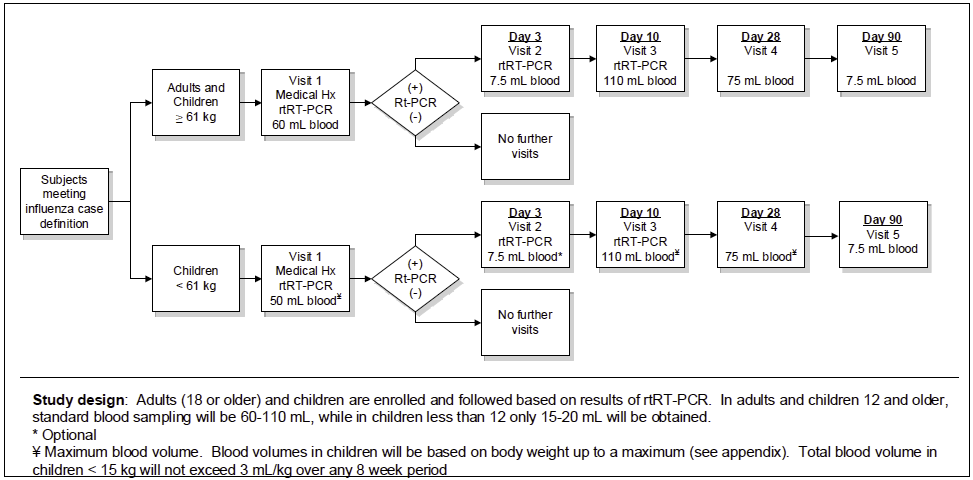
Population: Individuals with acute respiratory illness meeting the case definition for influenza (fever or feverishness accompanied by cough or sore throat) who have been symptomatic for 96 hours or less are eligible to participate in this study. We anticipate enrollment of approximately 20 to 30 subjects per influenza season. Over the course of 5 years, no more than 150 subjects will be enrolled.
Objectives: The objectives of this protocol are:
- To obtain timed samples of blood and nasal secretions from well characterized cases of acute influenza in support of research on the pathogenesis and host response.
- To evaluate the contribution of microevolution and antigenic drift to failed antibody-mediated protection.
- To compare host immunoglobulin adaptation to HA during infection and vaccination and characterize the mechanisms of immune escape during epidemic influenza infections in the young and old.
- To determine whether influenza-specific CD4 T cells are modified by infection and if so, how the strain of infecting virus influences CD4 T cell functionality.
- To define molecular mechanisms of innate immunity and molecular correlates of robust immune responses and protection following infection.
Study Design: The study will be conducted as a prospective study according to the schema outlined in the figure below:
Title: Collection of Blood and Body Fluids from Healthy Donors for Assay Development for the New York Influenza Center of Excellence
Population: Up to 800 normal, healthy subjects between the ages of 1 month and 80 years of age may participate in the study.
Participant Duration: Up to 1 hour
Objectives: The goal of this protocol is to provide serum, peripheral blood mononuclear cells, respiratory secretions and other body fluids from healthy donors for the development of assays related to the study of influenza and to assist the laboratories in quality control activities.
Schematic of Study Design: The study consists of a single visit where a single venipuncture and/or collection of other body fluid will be collected. However, individual subjects may participate more than once. If blood is collected adults may participate again as long as they do not donate more than 450 mL of blood total within any 56-day period including study blood samples and blood donations for subsequent transfusion use. Subjects 6 months up to 8 years do not donate more than 1 ml per Kilo of body weight, but less than 50mL, and subjects 8 years old up to 18 years old do not donate greater than 50mL total in a 56 day per
Title: Evaluation of the effects of age, prior exposure and previous vaccination on the B cell response to inactivated influenza vaccine in healthy adults and children.
Population: 240 Healthy adults and children ages 9 and over
Objectives:
Primary Objective
To evaluate the relationship between first influenza A virus exposure (inferred by age), vaccine history, and baseline serum antibody and Memory B cell (MBC) specificity, and the magnitude and breadth of the subsequent B cell response to seasonal influenza vaccine in healthy adults and children.
Primary Endpoint:
- Frequency and specificity of peripheral blood antibody secreting cells (ASC) on day 7 post vaccination (initial response), overall and stratified by age group, vaccine history, and baseline serum antibody level
- Frequency and specificity of peripheral blood memory B cells (PMBC) on day 28 post vaccination (memory response), overall and stratified by age group, vaccine history, and baseline serum antibody level
- Measurement of the magnitude and specificity of the serum antibody responses by hemagglutination (HAI), microneutralization (MN), genome fragment phage display (GFPD), surface plasmon resonance (SPR), and Hemagglutinin Array assays at day 7 and 28, overall and stratified by age group, vaccine history, and baseline serum antibody level.
Secondary Objectives
Determine the durability and breadth of the antibody response and relationships between acute and durable antibody
Secondary Endpoints
- Serum antibody response on day 90 after vaccination by HAI, MN, GFPDL, SPR and by Hemagglutinin Arrays, overall and stratified by acute antibody response
Exploratory Objective
Evaluate factors related to failure of vaccine to provide protection against symptomatic influenza and the immune response to infection in vaccinated individuals by prospective surveillance of the vaccine cohort.
Exploratory Endpoints
- Description of demographic and clinical characteristics and immune response to vaccination among subjects who develop symptomatic influenza (Vaccine Failures) and among subjects who do not.
- Descriptive comparison of the immune response to infection (Study DMID 14-0101) with the response to vaccination.
Schematic of Study Design:
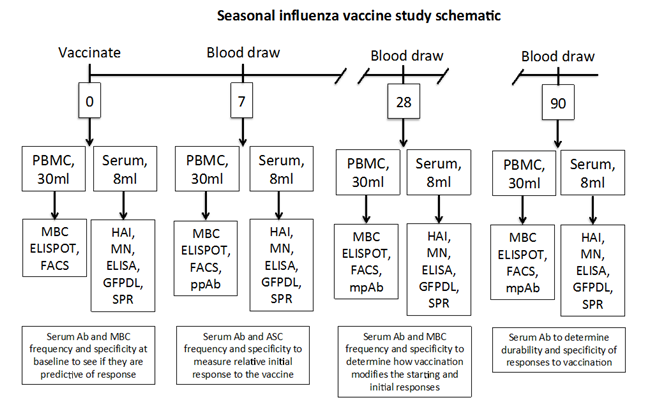
* Note: For children weighing less than 100 lbs, only 15 mL of blood will be drawn at each time point.and plasma will be used for antibody testing. Subjects will be queried regarding any blood being drawn for other purposes such as clinical testing, and that the total drawn will not exceed the above amounts
In addition, beginning on day 48, subjects will be contacted by phone once every two weeks during the influenza season and asked about influenza symptoms. Subjects who report symptoms will be asked to return for a clinic visit and specimen collection. Influenza season will be defined as when community laboratories detect 4 or more positives in any one week for two weeks in a row
Title: A comparison of CD4 T cell induction and antibody responses between a pure hemagglutinin influenza vaccine (rHA, Protein Sciences Corp) and licensed subvirion influenza vaccine made in eggs (Sanofi) or cell culture (Novartis) in healthy adults.
Population: Healthy adults ages 18 to 49 years
Agent description: Flublok (baculovirus exressed HA), Flucelvax (inactivated vaccine generated in mammalian cell culture, Fluzone SD (inactivated vaccine generated in eggs, administered at 15 ug per HA), Fluzone HD (inactivated vaccine generated in eggs, administered at 60 ug per HA)
Objectives:
Primary Objective
Comparison of the HA- specific CD4 T cell response in adults receiving an HA only vaccine to those in adults receiving egg- or cell-derived split or subvirion vaccines.
Primary Endpoint:
- CD4 T cell reactivity to pools of unique, conserved, and total pHA peptides derived from the pH1N1 HA, as well as H3, influenza B HA, NP, and M1 peptides will be quantified on days 0, 7, 14, 28 and 180 using Elispot assays and flow cytometry.
Secondary Objectives
- Comparison of the serum antibody response to immunization with pure HA, egg derived subvirion vaccine, or cell culture derived subvirion vaccine
- Comparison of the epitope specificity of B cells following vaccination with HA or subvirion vaccines
Schematic of Study Design:
Because it is possible that prior exposure will impact the relative impact of peptide specific help on the antibody response, subjects will be screened for pre-vaccination antibody. The vaccines will be trivalent and, subjects will be divided into relatively high (greater than 1:8) or low (equal to or less than 1:8) pre-vaccination antibody based on pH1N1. The basic study design is described below
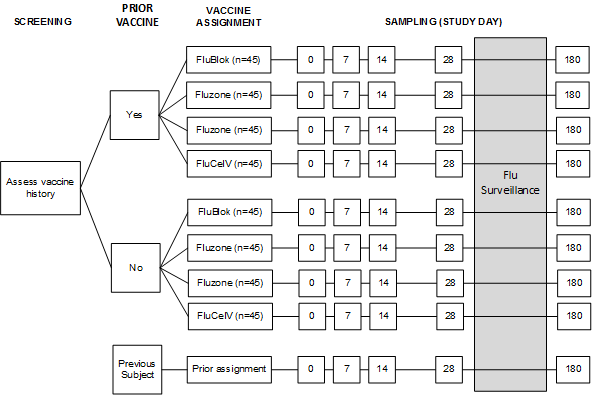
Subjects will be asked regarding prior history of influenza vaccination, and stratified into those who have not been vaccinated in the previous year and those who have, followed by randomized assignment to receive one of four licensed vaccines. Study subjects who participated in a previous year can be re-enrolled and will receive the same vaccine they received previously. Because the goal of the protocol is to evaluate the immune response to the vaccine and is not intended as a formal comparison of the adverse event profile, the vaccine assignment is not blinded. Following vaccination, subjects will have samples of blood obtained at the time points illustrated above. The last study visit will take place approximately 6 months after vaccination in order to test the durability of the response.