Projects
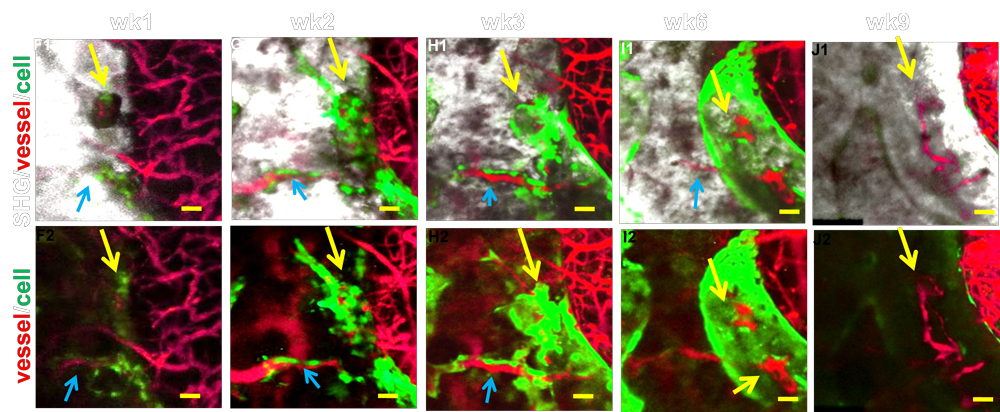
Blood vessel specification at the osteogenic and angiogenic interface in cranial bone tissue engineering.
Angiogenesis is a key component in bone repair and bone tissue engineering. While extensive research has demonstrated the interdependent role of osteogenesis and angiogenesis in repair and regeneration, little is known about how functional blood vessel networks are organized to initiate and facilitate bone tissue repair and regeneration. A functional blood vessel network consists of arteries, veins and a capillary interface that connects arterial and venous vessels for proper vascular perfusion. Establishing an organized expansion of these functional networks is essential for repair and regeneration. To understand angiogenesis at the site of bone repair and regeneration, we established a series of novel imaging approaches that permit high resolution, quantitative, and functional analyses of capillary vessels that couple to Col (I) 2.3 GFP+ osteoblasts at a cranial bone defect site. These approaches include 1) a cranial defect window chamber model that permits real-time, longitudinal, and functional assessment of angiogenesis coupling to bone regeneration via multiphoton-laser scanning microscopy (MPLSM); 2) an intravital imaging approach for probing tissue oxygenation and cellular metabolism at the repair site via two-photon phosphorescence/fluorescence lifetime imaging (2PLM and 2P-FLIM); and 3) a nanofiber-based tissue engineering approach to repair cranial bone defects. The goal of this proposal is to identify key regulatory elements of vessel specification under hypoxia in the bone tissue engineering microenvironment and further utilize the obtained information to establish novel strategies for improved bone regeneration.
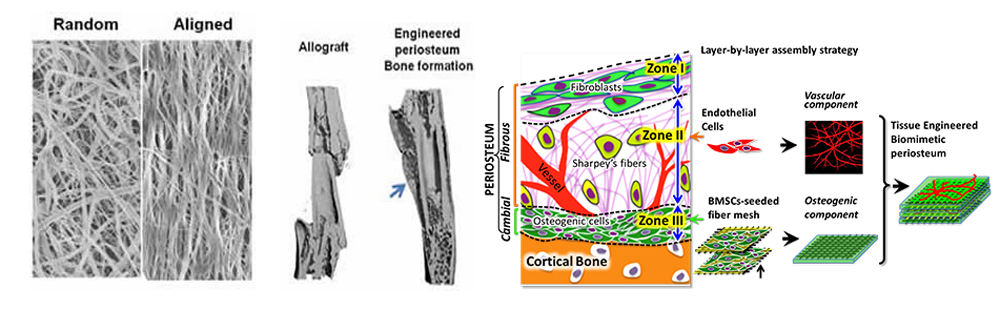
Engineering of Vascularized Biomimetic Periosteum for Bone Graft Repair and Reconstruction
Electrospun nanofiber holds great potential in tissue repair and regeneration due to its versatility in creating a scaffolding platform that allows presentation of integrated topographical and biochemical signals that are essential for stem cell manipulations. With the support from the University of Rochester Clinical and Translational Sciences Institute (UR CTSI), we have established a magnetic field-assisted electrospinning (MFAES) technique that allows the production of nanofibers with improved control of diameter, uniformity, and orientation. In view of the essential role of periosteum in bone tissue repair and regeneration, our current project proposes to combine nano- and microfiber technologies to create a multi-modular, prevascularized bone tissue graft, with growth factor releasing property, simulating the highly organized and functional periosteum for reconstruction of large bone defects. The completion of the project will establish a novel strategy to enhance vascularization of engineered bone constructs; further offering a tissue engineering solution and a translational therapeutic strategy to enhance bone defect repair and reconstruction.
Molecular Controls of Periosteum-mediated Repair
Periosteum is a microvascularized connective tissue that covers the outer surface of cortical bone. Periosteum contains abundant stem/progenitor cells that are essential for bone repair and regeneration. While the critical role of periosteum in bone repair has been well established, the molecular pathways that control periosteum-mediated repair and regeneration remain superficially understood. We have established a segmental bone graft transplantation model that allows molecular analyses of periosteum contribution to bone repair and reconstruction. By transplanting a LacZ marked bone grafts from R26A mice into a wild type recipient mouse, we tracked periosteal cell fate during graft healing and show that the expansion and further differentiation of the progenitor cells account for about 70% of bone and cartilage formation during the initiation stage of healing. The removal of the donor periosteum results in marked impairment of bone graft healing whereas the engraftment of multipotent mesenchymal stem cells (MSCs) on acellular bone allografts markedly improves healing and graft incorporation. These studies underscore the critical role of periosteal MSCs in repair and reconstruction, providing a strong rationale for a deeper understanding of the molecular signals that control proliferation and differentiation of periosteum derived MSCs in repair and reconstruction. Our current project centers on a number of integrated molecular signaling pathways that are critical for periosteum-initiated bone repair. By understanding these signals, our goal is to be able to establish targeted-therapeutic approaches to enhancing repair and reconstruction.
Tissue material interactions during repair and regeneration
Both biomaterials and allografts can trigger a host immune reaction that leads to fibrotic tissue formation and implant failure. While studies have suggested a role of macrophages and TGF-β signaling in the process, so far no anti-TGF-β strategy has been reported. A significant amount of literature has demonstrated a reciprocal and opposing relationship between TGF-b signaling and BMP signaling in control of the differentiation of osteoblasts and chondrocytes. The antagonisms between the two signaling pathway has also been suggested in the clinical context of muscle regeneration and treatment of fibrosis in various tissues. However, the reciprocal regulation between TGF-β and BMP-2 signaling has not yet been explored in bone healing and implant-associated fibrosis. The primary objective of this proposal is to determine the effects of simultaneous and sequential delivery of a BMP-2 mimicking peptide and an anti-fibrotic TGF-β signaling inhibitor via allograft surface coating on promoting bone formation and inhibiting fibrotic response in defect repair and reconstruction.