Genetic Mechanisms in Susceptibility to Noise Damage
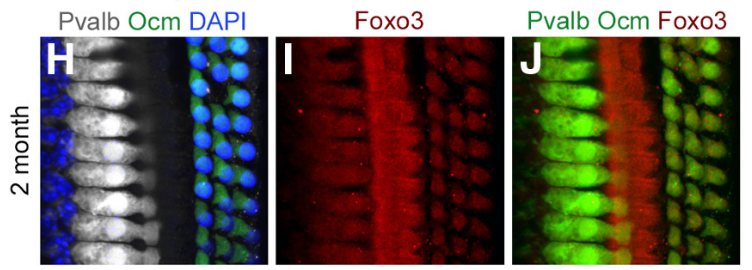
Image from Gilels et al. 2013
Whole-mount preparation of the 30 kHz region of a 2-month-old cochlea stained with anti-parvalbumin (white, IHCs), anti-oncomodulin (green, OHCs), and DAPI (blue, all nuclei). I, Anti-Foxo3 (red) labels hair cells and pillar cells. J, Colocalization of H with I; both hair cell markers are rendered in green and anti-Foxo3 staining is in red.
There is a wide variation in individual susceptibility to noise damage among workers experiencing a similar acoustic environment. Some of this variation can be attributed to differences in genes. We are interested in identifying genes that are necessary to restore cochlear function after noise damage. Our ultimate goal is to understand how noise damages cochlear cells, driving death and dysfunction, as a necessary first step in hearing restoration.
We have determined that the transcription factor FOXO3 plays a key role in maintaining cochlear cells’ survival and function after noise exposure. FOXO3 regulates a variety of cellular processes, including apoptosis, the oxidative stress response, autophagy, and proliferation. Recently, other groups have linked human variants in FOXO3 to susceptibility to hearing loss from industrial noise, supporting a role for this protein in human disease. Mice that lack FOXO3 protein develop severe hearing loss after exposure to noise that only causes temporary dysfunction in their wild-type littermates. In the FOXO3-KO, higher-frequency outer hair cells, resident in the base of the cochlea, die rapidly, 4 - 24 hours after noise exposure. Mid and lower frequency outer hair cells survive, but have reduced function and in some cases persistent damage to their stereocilia. Lost outer hair cells appear to be engulfed by their neighboring supporting cells; experiments to better define the manner and time course of cell death are ongoing.
These experiments show that FOXO3 is important in hearing preservation. However, it is unclear whether FOXO3 acts directly in outer hair cells to preserve their function, or indirectly in supporting cells. In order to define the mechanisms through which FOXO3 acts, we are performing cell-specific FOXO3 deletion experiments using inducible CRE recombinase (iCre) technology. In those experiments, FOXO3 is conditionally deleted, i.e. mutated, in either outer hair cells or supporting cells. We are comparing noise susceptibility for cell-specific FOXO3 deletions by mating a floxed-Foxo3 mouse line to a Prestin-iCRE or an Fgfr3-iCre, respectively. Once generated, these mice will be exposed to noise. After we have determined which cells must express FOXO3 to preserve outer hair cells and cochlear function, we will use RiboTag technology to determine how the translatome differs between FOXO3-KO cells and control cells.