Platelet Lysate (PL) Product As a New Means For Topical Application in Wounds
We obtained a patent for developing a new, procoagulable, platelet lysate product to be potentially administered topically for bleeds in both trauma and surgical settings. This product now is referred to as “Platelet Modified Lysate” (PML). We are using thromboelastography (TEG), platelet and whole blood aggregation studies, thrombin generation assay (TGA), as well as flow cytometry to evaluate our product’s effectiveness compared to standard platelet products.
Below is a plot of TGA tracings of platelet lysate compared to stored platelets and normal donor platelet rich plasma (PRP). In conjunction with other studies, we have demonstrated that PML is more stable and procoagulant than other blood products typically used to treat bleeding.
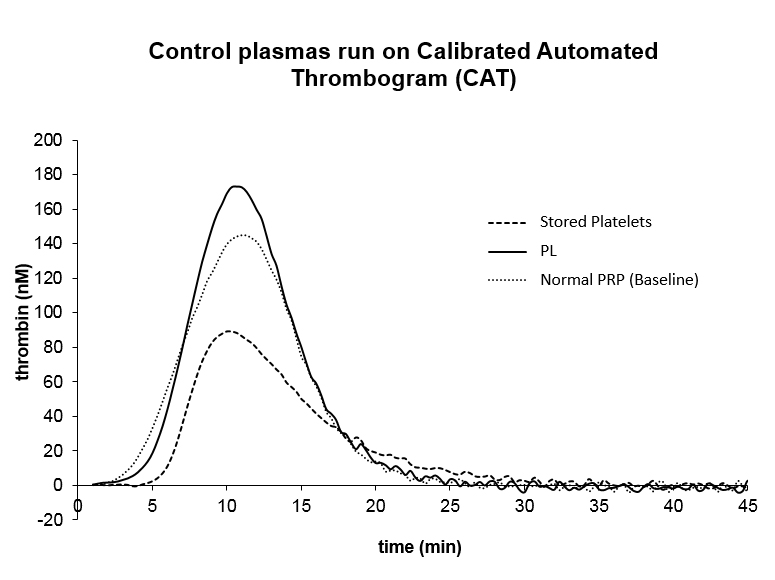
To read more about our in vitro studies, please refer to our paper titled “Evaluation of the procoagulant properties of a newly developed platelet modified lysate product”.
Currently, we are developing in vivo studies with liver injury models in mice.