Projects
Project 1: Resolution of neutrophil response for effective T cell functions and tissue repair (PI: Minsoo Kim)
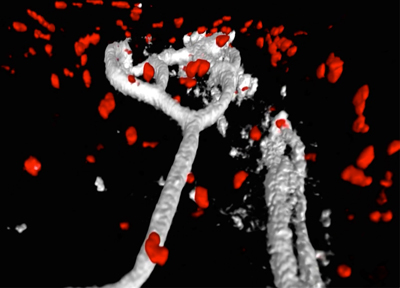 Migration of neutrophils to sites of tissue infection is vital for pathogen clearance and, thus, host survival. Importantly, once they complete their action, infiltrated neutrophils during the early infection phase should quickly initiate spontaneous apoptosis. A delayed neutrophil resolution is often associated with widespread tissue damage, organ failure, and ultimately death in severely infected patients. Therefore, the presence of unresolved neutrophil response during infection has long been believed to be detrimental, making it more difficult for patients to recover. Although growing evidence now suggests that neutrophils not only contribute to the tissue damage, but also orchestrate T cell functions and tissue repair, it has been challenging to clearly differentiate host-protective roles of neutrophils from their damaging inflammatory functions because most of the studies used over-activated or inappropriate inflammatory responses, which fail to restore tissue homeostasis. Therefore, the contribution of neutrophils to the host immune response and tissue repair may not be properly evaluated in these models. We undertook this study to address critical knowledge gaps regarding the function and fate of neutrophils during the infection. Through several lines of evidence from our preliminary study using a fully recovering mouse influenza infection model, we propose novel functions of neutrophil that can actively promote tissue healing and the resolution of inflammation. We discovered that apoptotic neutrophils actively engage with the proliferation and survival of other surrounding cells (e.g., epithelial cells and macrophages). This mitogenic and pro-survival effect of apoptotic neutrophils was mainly mediated by the secretion of epidermal growth factor (EGF). Moreover, live intravital multiphoton microscopy (IV-MPM) of our newly generated Ly6GCre/ROSAtdTomato/Csf1r-EGFP mice revealed a striking motility pattern of neutrophils and their phagocytes during the resolution phase within the infected airway, which may represent additional beneficial functions of neutrophils in protecting the host against infections. We hypothesize that resolution of neutrophil response during influenza infection is not merely a passive termination of the early neutrophil infiltrates but rather an active biochemical and cellular process to establish effective T cell immune responses while enabling tissues to repair and return to normal function. We will test the hypothesis that (1) apoptotic neutrophils promote tissue repair and (2) neutrophils control their own clearance. We will also determine (3) whether neutrophil resolution regulates T cell functions and memory formation. Given the important immune-modulatory properties of neutrophils, understanding of novel functions of neutrophil during the resolution of inflammation could be harnessed to combat infection and chronic inflammatory conditions.
Migration of neutrophils to sites of tissue infection is vital for pathogen clearance and, thus, host survival. Importantly, once they complete their action, infiltrated neutrophils during the early infection phase should quickly initiate spontaneous apoptosis. A delayed neutrophil resolution is often associated with widespread tissue damage, organ failure, and ultimately death in severely infected patients. Therefore, the presence of unresolved neutrophil response during infection has long been believed to be detrimental, making it more difficult for patients to recover. Although growing evidence now suggests that neutrophils not only contribute to the tissue damage, but also orchestrate T cell functions and tissue repair, it has been challenging to clearly differentiate host-protective roles of neutrophils from their damaging inflammatory functions because most of the studies used over-activated or inappropriate inflammatory responses, which fail to restore tissue homeostasis. Therefore, the contribution of neutrophils to the host immune response and tissue repair may not be properly evaluated in these models. We undertook this study to address critical knowledge gaps regarding the function and fate of neutrophils during the infection. Through several lines of evidence from our preliminary study using a fully recovering mouse influenza infection model, we propose novel functions of neutrophil that can actively promote tissue healing and the resolution of inflammation. We discovered that apoptotic neutrophils actively engage with the proliferation and survival of other surrounding cells (e.g., epithelial cells and macrophages). This mitogenic and pro-survival effect of apoptotic neutrophils was mainly mediated by the secretion of epidermal growth factor (EGF). Moreover, live intravital multiphoton microscopy (IV-MPM) of our newly generated Ly6GCre/ROSAtdTomato/Csf1r-EGFP mice revealed a striking motility pattern of neutrophils and their phagocytes during the resolution phase within the infected airway, which may represent additional beneficial functions of neutrophils in protecting the host against infections. We hypothesize that resolution of neutrophil response during influenza infection is not merely a passive termination of the early neutrophil infiltrates but rather an active biochemical and cellular process to establish effective T cell immune responses while enabling tissues to repair and return to normal function. We will test the hypothesis that (1) apoptotic neutrophils promote tissue repair and (2) neutrophils control their own clearance. We will also determine (3) whether neutrophil resolution regulates T cell functions and memory formation. Given the important immune-modulatory properties of neutrophils, understanding of novel functions of neutrophil during the resolution of inflammation could be harnessed to combat infection and chronic inflammatory conditions.
Project 2: Spatial optimization of T cell activation at inflamed sites via cytokine/chemokine-dependent cellular clustering (PI: Deborah Fowell)
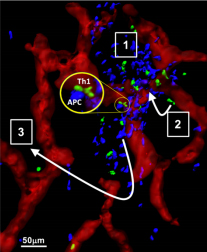 Spatiotemporal control of effector T cell activation at sites of inflammation/infection is an essential yet poorly understood process. Most intravital imaging studies have concluded that T cells scan inflamed tissues in a random non-directional fashion. Therefore, how CD4+ T cells ultimately position themselves for effective antipathogen immunity remains elusive. For Th1 effectors the optimal location of effector T cell activation is likely dependent on the active range of secreted effector molecules such as IFNg, estimated to be ~80 microns in cutaneous Leishmania major infection. Despite knowing some of the key cellular and molecular players essential for T cell accumulation at sites of inflammation, how they are spatially and temporally positioned and released remains a critical knowledge gap that hinders new approaches to therapeutic manipulation to enhance immunity to infection and to diminish autoimmune tissue damage. Using CXCL9/10 fluorescent reporter mice to visualize the cellular source/location of chemokine production and intravital-multiphoton microscopy (IV-MPM) to track Th1 migration, we found chemokine producing cells were spatially restricted to perivascular clusters (PVC) that were enriched in MHC-IIhigh antigen presenting cells and that shaped the localization and motility of Th1 cells in the inflamed/infected dermis (see Model). Our overall hypothesis is that initial peripheral activation occurs in chemokine-rich peri-vascular clusters that serve to nucleate and amplify T cell recruitment and activation for efficient pathogen clearance. This nucleation step may facilitate efficient pathogen clearance but may also exacerbate the magnitude of immune damage in autoimmune settings. This proposal uses IV-MPM and photoactivation tools for spatiotemporal dissection of the organization, composition and impact of these chemokine ‘hubs’ on Th1 activation and their role in optimizing protective immunity at foci of infection. Aim 1. Organization of chemokine-rich perivascular clusters via innate cell:Th1 cross-talk. This Aim uses the spatial precision of photoactivation (PA-GFP) to define the cellular composition of the chemokine-rich PVCs, determines the critical innate cellular players in promoting Th1 activation within the clusters and tests the hypothesis that these initial chemokine-rich PVCs serve to activate early Th1 ‘pioneers’ entering the tissue and that cytokines released by those Th1 cells drive a local positive amplification loop to boost subsequent Th1 cell recruitment and/or activation. Using cell type-specific deletion of the IFNgR we will identify the responding innate cell types critical in driving this amplification loop. Aim 2. Functional impact of T cell activation within the clusters. We hypothesize that the positioning of both chemokine producing cells and antigen presentation within the PVCs serves to nucleate signals for efficient Th1 activation. In turn, cytokines/chemokines produced by Th1 cells locally within the PVC serve to boost APC function. In this Aim, we seek to determine whether activation within the PVC confers distinct functional advantages to Th1s and to identify the MHC-II+ cell types that confer such benefits. We will use IVMPM analysis of T:APC interactions within and outside the chemokine-rich PVC. We will also employ the PAGFP system to spatially define Th1 cells in the inflamed tissue to determine if PVC location and/or activation therein drives a distinct functional program. Aim 3. Relationship between peri-vascular clusters and the infection foci. Chemokine-rich perivascular clusters containing Th1 cells can be found 100-400µm from the antigen depot or from the site of primary infection. We hypothesize that early PVC nucleation is followed by local diaspora of activated Th1 cells that accumulate at infection foci. This possible 2-step process within inflamed tissues would facilitate initial Ag-dependent activation peri-vascularly with subsequent re-positioning to the site of pathogen infection for antimicrobial effector function. To test our hypothesis, we will use two cutaneous infection models, L. major and OVA-expressing vaccinia virus (VV) to assess Th1 exchange between PVC and infection foci.
Spatiotemporal control of effector T cell activation at sites of inflammation/infection is an essential yet poorly understood process. Most intravital imaging studies have concluded that T cells scan inflamed tissues in a random non-directional fashion. Therefore, how CD4+ T cells ultimately position themselves for effective antipathogen immunity remains elusive. For Th1 effectors the optimal location of effector T cell activation is likely dependent on the active range of secreted effector molecules such as IFNg, estimated to be ~80 microns in cutaneous Leishmania major infection. Despite knowing some of the key cellular and molecular players essential for T cell accumulation at sites of inflammation, how they are spatially and temporally positioned and released remains a critical knowledge gap that hinders new approaches to therapeutic manipulation to enhance immunity to infection and to diminish autoimmune tissue damage. Using CXCL9/10 fluorescent reporter mice to visualize the cellular source/location of chemokine production and intravital-multiphoton microscopy (IV-MPM) to track Th1 migration, we found chemokine producing cells were spatially restricted to perivascular clusters (PVC) that were enriched in MHC-IIhigh antigen presenting cells and that shaped the localization and motility of Th1 cells in the inflamed/infected dermis (see Model). Our overall hypothesis is that initial peripheral activation occurs in chemokine-rich peri-vascular clusters that serve to nucleate and amplify T cell recruitment and activation for efficient pathogen clearance. This nucleation step may facilitate efficient pathogen clearance but may also exacerbate the magnitude of immune damage in autoimmune settings. This proposal uses IV-MPM and photoactivation tools for spatiotemporal dissection of the organization, composition and impact of these chemokine ‘hubs’ on Th1 activation and their role in optimizing protective immunity at foci of infection. Aim 1. Organization of chemokine-rich perivascular clusters via innate cell:Th1 cross-talk. This Aim uses the spatial precision of photoactivation (PA-GFP) to define the cellular composition of the chemokine-rich PVCs, determines the critical innate cellular players in promoting Th1 activation within the clusters and tests the hypothesis that these initial chemokine-rich PVCs serve to activate early Th1 ‘pioneers’ entering the tissue and that cytokines released by those Th1 cells drive a local positive amplification loop to boost subsequent Th1 cell recruitment and/or activation. Using cell type-specific deletion of the IFNgR we will identify the responding innate cell types critical in driving this amplification loop. Aim 2. Functional impact of T cell activation within the clusters. We hypothesize that the positioning of both chemokine producing cells and antigen presentation within the PVCs serves to nucleate signals for efficient Th1 activation. In turn, cytokines/chemokines produced by Th1 cells locally within the PVC serve to boost APC function. In this Aim, we seek to determine whether activation within the PVC confers distinct functional advantages to Th1s and to identify the MHC-II+ cell types that confer such benefits. We will use IVMPM analysis of T:APC interactions within and outside the chemokine-rich PVC. We will also employ the PAGFP system to spatially define Th1 cells in the inflamed tissue to determine if PVC location and/or activation therein drives a distinct functional program. Aim 3. Relationship between peri-vascular clusters and the infection foci. Chemokine-rich perivascular clusters containing Th1 cells can be found 100-400µm from the antigen depot or from the site of primary infection. We hypothesize that early PVC nucleation is followed by local diaspora of activated Th1 cells that accumulate at infection foci. This possible 2-step process within inflamed tissues would facilitate initial Ag-dependent activation peri-vascularly with subsequent re-positioning to the site of pathogen infection for antimicrobial effector function. To test our hypothesis, we will use two cutaneous infection models, L. major and OVA-expressing vaccinia virus (VV) to assess Th1 exchange between PVC and infection foci.
Project 3: Formation, Positioning, Motility, and Function of Tissue Resident Memory CD8+ T cells After Influenza Infection” (PI: David Topham)

Tissue resident memory T cells (TRM) are non-recirculating CD8+ T cells that become established in peripheral tissues after an infection. Upon re-encounter with the same or related pathogen(s), these memory T cells rapidly reactivate and provide immediate effector function that can mean the difference between life and death in a lethal challenge model. They tend to be specific for conserved antigens, and in the case of influenza, could be part of the solution to achieving more broadly cross-reactive and universal immunity. Understanding how they are regulated, how they mediate optimal protection, and how they are established and maintained are critical goals of this project. Besides the markers used to identify the T cell types (CD3, CD8, CD44, CD62L, CD69), several other cell surface markers are commonly used to identify memory T cell subsets in the tissues. CD49a, when paired to integrin beta-1 to form VLA-1, is a receptor for collagen in the extracellular matrix and is the prototypic TRM marker first used to define these cells in the tissue by us in 2004. Blockade or deletion of CD49a leads to loss of TRM in the periphery and loss of heterosubtypic immune protection. CD103, when paired with beta-7 integrin, binds to E-cadherin expressed in the junctions between epithelial cells. CD103 has been more commonly used than CD49a to mark TRM, but as we will show, only identifies a subset of these cells giving an incomplete picture. The majority of TRM express CD49a, with about 30% of those co-expressing CD103. Using CD49a and CD103, four subsets of memory CD8 T cells can be identified in the lung. Each has distinct gene expression and effector functions. Markers are all well and good, but do not tell us what their function is. Little has been done to determine the functions of CD49a and CD103 besides some deletion or inhibition studies followed by a census of the cells remaining in the tissues. Our research revealed that CD49a is needed for locomotion of TRM in the tissue, while CD103 acts to tether the cells to epithelial cell junctions. We are testing hypotheses related to how each of these adhesion molecules acts to position memory T cells in different anatomical locations, regulate communication with the epithelium, are associated with genetic programming linked to functional differentiation, thereby regulating CD8+ T cell motility, survival, and optimal immune protection.
Aim 1: Determine the mechanisms that determine differentiation, establishment, and maintenance of TRM subsets after influenza infection. We have used flow cytometry of the integrins CD49a and CD103 to define four different subsets of memory CD8 T cells found in the airways and lung tissue. RNAseq data shows tight clustering of each of these subsets, suggesting there are transcriptional and epigenetic changes that differentiate them. Knowing how TRM subsets are genetically regulated may lead to strategies to enhance their generation and maintenance, which could improve cross-protective immunity to influenza. In this Aim we will test the hypothesis that signals from the airway and lung environments during influenza infection drive permanent changes in gene expression partially mediated through specific transcription factors and epigenetic modifications.
Aim 2: Investigate mechanisms of T cell-epithelial cell-matrix interactions required for motility and positioning in the airways. Sensing changes in the tissue microenvironment and responding to cues are critical for memory T cells to provide immune surveillance and protection. Integrins and other adhesion molecules mediate T cell interaction and communication with the environment and their ability to migrate and position optimally in the tissue. Our preliminary data show that CD49a supports locomotion on collagen and is critical for the maintenance of TRM. CD103 supports tethering to E-cadherin expressed by epithelial cells and is important for the accumulation of CD8 T cells in the airways. Imaging has also revealed close association of OT-I T cells with adjacent epithelial cells, and novel preliminary RNAseq data has identified expression of three tight junction genes by the CD49a+ CD103+ TRM. Together, the hypothesis is formed that CD8 TRM motility and surveillance in the epithelium involves active formation of junctions with epithelial cells via CD103/E-cadherin and other junctional proteins, and locomotion on collagen. These interactions are expected to specifically position the cells in the epithelium and regulate immune surveillance.
Aim 3: Determine the functions of CD49a and CD103 in optimizing immune protection. Preliminary data demonstrates that TRM from the respiratory tract can be divided into four subsets based on integrin expression, and we see further functional differences in each of the four subsets, with differential effector cytokine secretion and cytotoxic potential. RNAseq data provides evidence for expression of junctional proteins, potentially providing other mechanisms that regulate CD8 TRM motility and positioning. This aim seeks to test whether the integrins CD49a and CD103 act in concert with junctional proteins to provide optimal immune surveillance and effector function upon secondary encounter with pathogen. In this Aim we seek to test this hypothesis through experimental interventions that perturb EC/T cell interactions, followed by secondary virus infection.
Project 4: Mechanics of T cell migration (PI: Patrick Oakes)
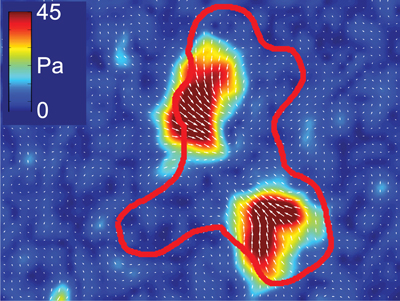 Migration is a fundamental component of a functional immune system. Responding to both chemical and mechanical signaling cues, cells need to move in complex environments including both within and between tissues. Cells move by generating internal forces which can be coupled to their surrounding matrix. If cells don’t generate any forces, or can’t adhere to other cells or proteins, they can’t move. Similarly ,too much adhesion will keep a cell stuck in place, and too much force generation will break adhesions before they can be used. Migration thus requires the delicate balance of adhesion and force generation in both space and time. While this balance has been extensively explored in the context of mesenchymal cell migration, these relationships remain ill-defined in the amoeboid migration modes used by T cells and other immune cells. Our overall goal is to define the mechanical interactions that enable and guide immune cell migration under different physiological conditions.
Migration is a fundamental component of a functional immune system. Responding to both chemical and mechanical signaling cues, cells need to move in complex environments including both within and between tissues. Cells move by generating internal forces which can be coupled to their surrounding matrix. If cells don’t generate any forces, or can’t adhere to other cells or proteins, they can’t move. Similarly ,too much adhesion will keep a cell stuck in place, and too much force generation will break adhesions before they can be used. Migration thus requires the delicate balance of adhesion and force generation in both space and time. While this balance has been extensively explored in the context of mesenchymal cell migration, these relationships remain ill-defined in the amoeboid migration modes used by T cells and other immune cells. Our overall goal is to define the mechanical interactions that enable and guide immune cell migration under different physiological conditions.
T cells lack both the actin stress fibers and large focal adhesion plaques typical of mesenchymal cells. Their motility is rather typified by what appear to be rapid polymerization of branched actin networks and transient interactions with the substrate. These features should make them particularly susceptible the mechanical properties of their surrounding mechanical environment. We hypothesize that the migration behaviors of different immune cells lie along a single continuum, differing only in their relative contributions of adhesion and force generation. We further speculate that effector programming leads to differences in the activation thresholds for these physical interactions that may modify the way distinct effector subsets respond to their physical microenvironment. To test this hypothesis, we propose to use Th1 and Th2 T cells in the following aims: Aim 1. To determine the relation between actin polymerization and traction stress in Th1 and Th2 cells; Aim 2. To determine how ECM composition, organization and material properties regulate adhesion; Aim 3. Do T cells adapt their migration behavior to the microenvironment in vivo. This research will provide a novel physical and mechanistic perspective for understanding how T cells move and how changing their physical interactions with their environment effects their function.