High-Throughput Imaging with Parallel Scanning Multiphoton Microscopy
High-Throughput Imaging with Parallel Scanning Multiphoton Microscopy
Multiphoton microscopy has been ubiquitously used for tissue imaging due to its resilience to light scattering and deep imaging depth. Developments in multiphoton microscopy over recent years have led to a significant increase in imaging throughput (up to 200 Megapixels per second) over conventional multiphoton microscopes (few Megapixels per second). However, these systems all focus on high-temporal resolution imaging of a single field-of-view and perform poorly with high-area imaging. Therefore, for fast imaging of large samples such as surgical excisions, other high-area rate fluorescence microscopy techniques such as light-sheet imaging, are used but consequently sacrifices advantages related to scattering, imaging depth, and potential for capturing nonlinear processes.
We leveraged our previous work with silicon photomultipliers (SiPM) into the development of a SiPM array detector, which allows for non-descanned detection of fluorescence parallel scanned foci at extremely high speeds (effective 200 Megapixels per second, or 800 Megaspectra per second with four channels) while capturing scattered light efficiently without significant scattering crosstalk between detectors. Combined with tilted-plane parallel strip-scanning, we achieve an effective area coverage rate of 50 mm2/s, which allows for multi-level imaging of a large surgical excision in under one minute.
Ongoing work in this project involves miniaturization of this technology onto a clinical cart system to perform on-site and/or intraoperative clinical studies.
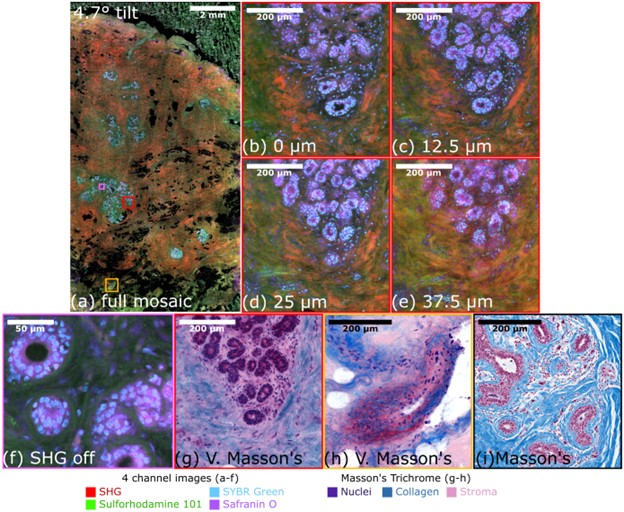
Figure 1: Four channel imaging of human breast tissue with parallel multiphoton microscopy. (a) The full mosaic of the whole excision at 0 µm. (b-e) Zoomed in region of a gland at multiple depths are shown. (f) A zoomed in region of another gland with SHG turned off demonstrating high SNR imaging. (g-h) Virtual Masson’s trichrome images of glands and vessels show potential for clinical pathology, (i) Example of a Masson’s trichrome for conventional histology.
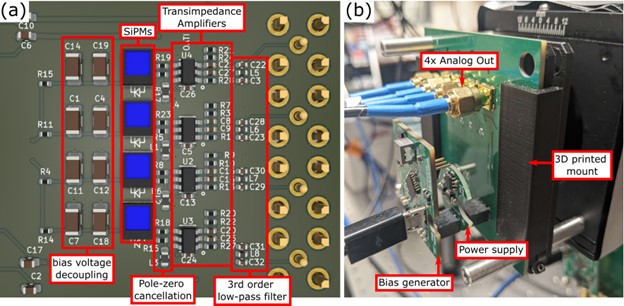
Figure 2: (a) The parallel scanning detector board consists of four SiPMs uniformly arrayed along with the corresponding transimpedance amplifiers, pole-zero cancellation filters, and third order low pass filters. (b) The same OpenSiPM bias generator and power supply boards are used by the detector board and are all mounted onto cage rod assemblies.