Research Projects
Development of a Novel Stimulator and Ultrasound Probe for Tissue Characterization of Congenital Hindgut Disorders
Congenital anomalies of the hindgut encompass a wide range of congenital malformations, including anorectal malformations and Hirschprung disease. These conditions involve the hindgut itself and the musculoskeletal tissues (e.g., sphincter and pelvic floor muscles) that are intimately associated. Most research has previously focused on the embryologic events that lead to these conditions and how best to treat them after birth. However, not much is known about the tissue characteristics of the hindgut and the associated musculoskeletal tissues in these conditions. The purpose of this research is to build a tool that will allow us to better understand these tissue characteristics using muscle stimulation, B-mode ultrasound, and ultrasound elastography.
Development of a Noninvasive Comprehensive Digital Tool for Modeling the Structure and Function of the Small Intestine
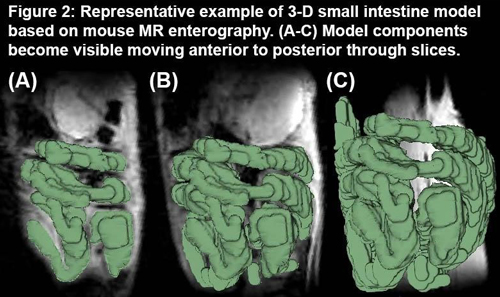
Small intestinal diseases affect patients throughout their entire lifespan. While the clinical burden of small bowel pathology is astonishingly high, there are no current methods to directly, noninvasively quantify small intestine function. Clinically, small intestine function is inferred using surrogate markers, but more direct assessment of the small intestine physical properties are achievable using novel quantitative techniques. Our overall purpose is to develop a validated, comprehensive digital tool that enables non-invasive, subject-specific measurements of small intestine physical properties. In patients with intestinal failure, this tool will allow us to predict patients’ ability to grow and thrive without parenteral nutrition and to predict whether small bowel anatomy is amenable to, and the likely success of, surgical procedures.
Decision Support Tool for Triage of Trauma Patients: Using Machine Learning to Take the Complexity out of Trauma Triage
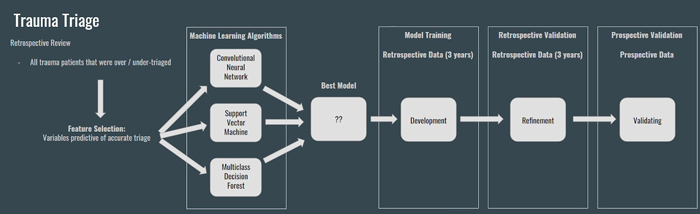
Approximately 10-15% of all traumatically injured children sustain life-threatening injuries that require a systematic and rapid approach to their treatment. When a trained trauma resuscitation team is present on the arrival of the seriously injured child, mortality can be decreased by 25%-30%. Undertriage is defined as a triage decision that classifies a patient as not requiring a trauma team activation, when in fact they do. Undertriage is a medical problem, which may result in adverse patient outcomes. Overtriage occurs when a trauma team activation is triggered despite the patient not meeting criteria for such an activation. Overtriage is a resource utilization problem. The American College of Surgeons views overtriage and undertriage rates based on trauma team activation criteria as surrogate markers for quality trauma patient care.
We are conducting exploratory studies aimed at demonstrating the feasibility of a non-invasive, population-based predictive model for trauma triage. Our ultimate goal is to develop a prospectively validated clinical decision support tool that can be used in conjunction with clinical judgement from human providers to make the best trauma triage decisions possible.
Using Fractals to Investigate How Tissue Structure and Organization Promote Vasculogenesis in 3D Engineered Tissues
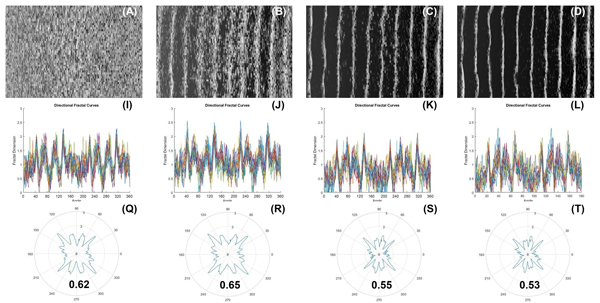
The fractal concept is a mathematical model initially used to characterize the complex geometry of nature, whose irregularity and complexity cannot be readily simulated using traditional linear geometry. We are currently using tissue engineering constructs, such as cellular arrays in hydrogels as an archetype system to demonstrate and illustrate self-organization and emergence, as characterized using fractal mathematics. We are also investigating how the organizational properties of these cellular constructs may affect vasculogenesis in 3D engineered tissues.
Use of Photodynamic Therapy to Treat Intra-Abdominal Contamination in General Surgical Conditions
Intra-abdominal contamination is common in a range of general surgical conditions, including appendicitis, perforated diverticulitis, and perforated gastric and duodenal ulcers. Despite surgical treatment for the primary condition, relatively long durations of parenteral antibiotic administration are also required to treat the resulting intra-abdominal contamination in these conditions. Long durations of parenteral antibiotic administration result in prolonged hospital stays and may not be effective against antibiotic-resistant bacterial strains, highlighting the need for alternative therapies. Appendicitis is an ideal model for intra-abdominal contamination because it is the most common abdominal surgical emergency worldwide, affects patients of all ages regardless of comorbidities, and there is an accessible animal model of perforated appendicitis. After appendectomy, parenteral antibiotic therapy remains the mainstay of treatment for intra-abdominal contamination associated with complicated appendicitis.
Photodynamic therapy (PDT) exerts antimicrobial activity through the generation of reactive oxygen species and is effective against a wide variety of microbial species. PDT is broad spectrum and fast acting (immediate vs. days/weeks for antibiotics), efficacious against antibiotic-resistant strains, and does not result in acquired resistance. Given that the intra-abdominal contamination associated with complicated appendicitis and other general surgical conditions is polymicrobial and often accompanied by bacterial resistance to common antibiotics, PDT is an appealing adjunct to the treatment of intra-abdominal contamination in general surgical conditions. Our aim is to demonstrate proof-of-concept for antimicrobial PDT as a treatment of intra-abdominal infection in complicated appendicitis and to demonstrate efficacy against bacteria isolated from human samples. This study could revolutionize the management of patients with complicated appendicitis and other general surgical conditions and reduce hospital stay, complications, and abscess development.
This work is being performed in conjunction with Dr. Tim Baran and the Baran Lab in Imaging Sciences and with Dr. Marty Pavelka and the Pavelka Lab in Microbiology.
Developing Novel Therapies to Prevent Post-operative Abdominal Adhesion Formation
Abdominal adhesions result when scar tissue forms between the abdominal wall and visceral organs following peritoneal tissue trauma during surgery. Adhesion formation occurs in more than 50% of cases, with incidence rising to 95% following multiple surgeries. Abdominal adhesions are the number one cause of small bowel obstruction, may lead to infertility and chronic pain, and can complicate subsequent surgeries. Collectively, United States healthcare costs associated with adhesion-related complications exceed $5 billion annually, and treatment options are limited to laparoscopic or open surgical lysis. Thus, there is a substantial need to identify therapies to prevent adhesion formation and to ameliorate adhesion persistence and recurrence. The central goal of this project is to assess the efficacy of novel anti-fibrotic therapies to prevent the formation and/or facilitate amelioration of post-operative intra-abdominal adhesions.
This work is being performed in conjunction with Dr. Alayna Loiselle and the Musculoskeletal and Soft Tissue Fibrosis and Regeneration Lab in the Center for Musculoskeletal Research and with Dr. Matt Kottmann and the Kottmann Lab in the Department of Medicine.
Understanding the Changes in Chest Wall Biomechanics with Pectus Excavatum and Pectus Carinatum
Pectus excavatum, pectus carinatum, and pectus arcuatum are three developmental chest wall disorders that present initially and then evolve during childhood. The exact etiologies of these disorders are unknown. Depression of the anterior chest wall is known as pectus excavatum (PE). PE is the most common chest wall deformity (90%) and occurs in approximately 1 in 400 births (0.25%). PE can range from mild to severe and in severe forms it can cause direct compression of the heart/lungs. Some research suggests that the changes in chest wall shape can result in changes in chest wall compliance, thereby further affecting growth and respiratory mechanics. Forward protrusion of the anterior chest wall is known as pectus carinatum (PC). PC is the second most common chest wall deformity and is ~5x less common that PE in North America. PC is also more common in males than females, with a ratio of 4:1. PC is not commonly associated with cardiopulmonary physiologic impairment. However, some patients report respiratory symptoms, which have been attributed to a relatively fixed chest wall with augmented residual lung volumes.
The purpose of this project is to develop a quantitative understanding of chest wall morphology and biomechanics in patients with chest wall deformities, including both pectus carinatum and pectus excavatum, using non-invasive 3D optical scanning and non-invasive force measurements. We hypothesize that chest wall deformities result in biomechanical changes to the thorax that are captured using non-invasive 3D optical scanning of the thorax. Understanding the 3D morphology and biomechanics of the thorax in patients with chest wall deformities will potentially suggest new ways to assess the severity of these deformities.