155+
Clinical Studies
45K+
Research Participants
Clinical Studies
Research Participants
UR Center for Health + Technology [CHeT]
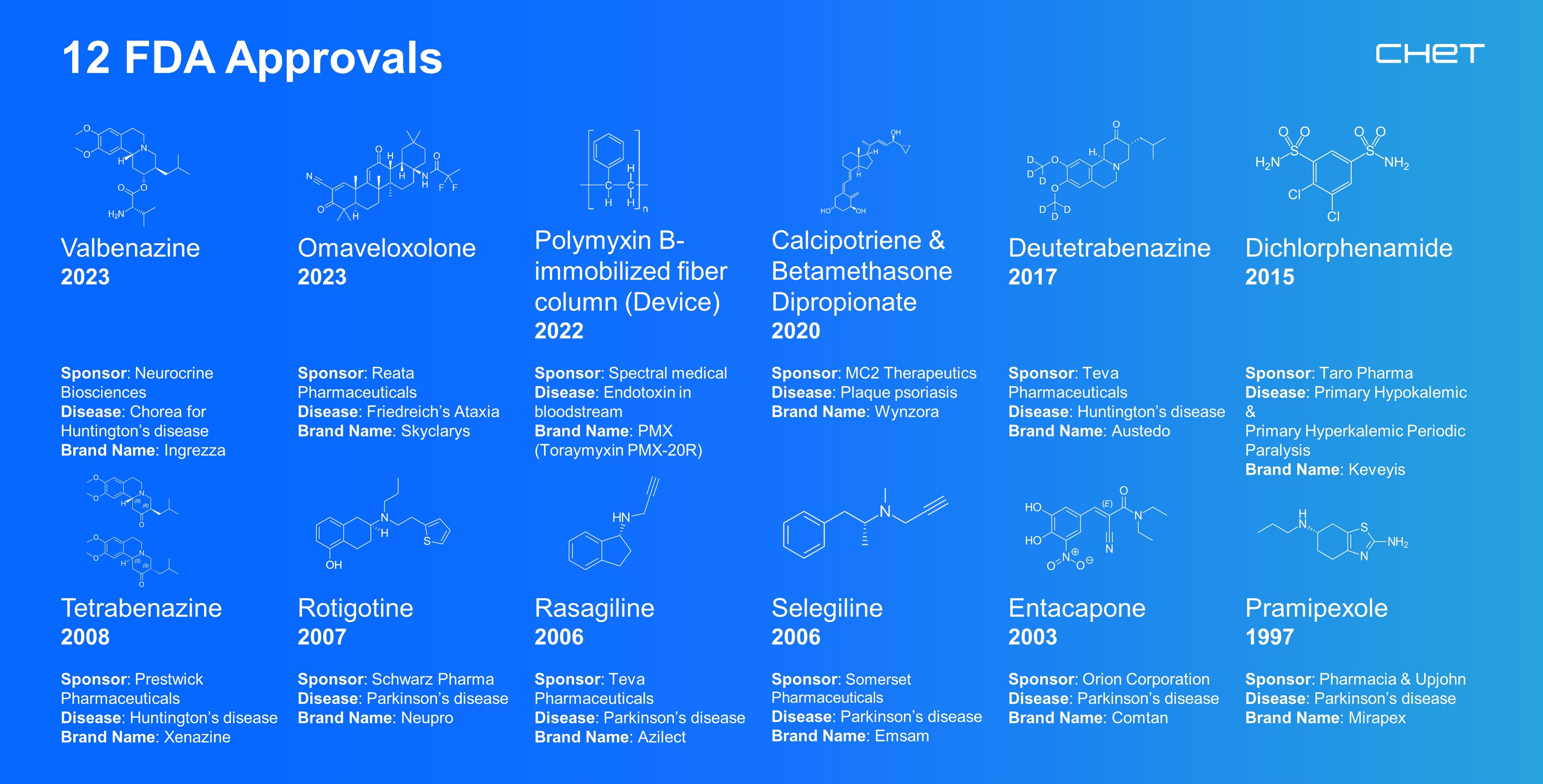
For more than three decades, CHeT’s clinical trials coordination center has served as a worldwide leader in the conduct, planning, management, implementation, analysis, and rescuing of large multi-center clinical research studies.
CHeT has six units with expertise in clinical trials coordination, clinical materials services, patient-reported outcome measures, tech research and innovation, data modeling and predictive analytics, and advancing health of our communities.
Our collaborators span the globe and include the National Institutes of Health, leading academic institutions, not-for-profit foundations, national advocacy groups, and industry innovators in pharma and technology.

The number of people affected by Parkinson disease has doubled over the past 25 years and is projected to double again in the next 25. The UR-Udall Center of Excellence is accelerating Parkinson disease research through disease modeling, virtual visits, and novel sensors at the University of Rochester.
Learn more about the UR-Udall Center
The Long Road to Hope, which shares the inspiring stories of 12 individuals living with Parkinson's, premieres on World Parkinson's Day, April 11. Watch the trailer.